|
 |
| Organoid > Volume 1; 2021 > Article |
|
Abstract
The blood-brain barrier (BBB) is a transport barrier that suppresses the translocation of potentially harmful substances to the brain tissue. Although the BBB is known to be associated with many kinds of neuropathology, such as neuroinflammation and neurodegenerative diseases, the conventionally used animal and Transwell models cannot provide sufficient information due to genetic and functional heterogeneity in comparison with humans and limited monitoring capabilities. Recently, human cell-based three-dimensional BBB models have been developed, and these models provide in vivo-like BBB structures and functions. In this review, we provide an overview of the recent advances in BBB models with a particular focus on the simulation of BBB-associated brain physiology and neuropathology. To this end, important factors for recapitulating the in vivo characteristics of the BBB are described. Furthermore, approaches to recapitulate the BBB physiology using engineering methods are summarized. The applications of BBB models in the study of neuropathology, such as inflammation and neurodegenerative diseases, are also presented.
The blood-brain barrier (BBB) is a functional unit in the blood vessels of the brain that regulates the transport of molecules between the circulating blood and brain tissue [1]. The BBB selectively transports molecules based on needs for brain homeostasis [2]. For example, water, gases, and lipid-soluble molecules are transported via passive diffusion, while glucose and amino acids that are required for metabolism enter the brain tissue via selective transport [3,4]. In contrast, neurotoxins and drugs are generally not allowed to pass the BBB and are pumped out from the perivascular region via active transport [5]. Consequently, the brain maintains homeostasis irrespective of the daily diet [6].
The BBB is mainly composed of 3 cell types—brain endothelial cells, pericytes, and astrocytes—and these cell types all contribute to BBB function. Endothelial cells form a tubular blood vessel structure with tight junctions [7]. Pericytes wrap around the brain microvasculature and promote BBB maturation and stability via both direct contact-mediated and paracrine-mediated mechanisms [8,9]. Astrocytes extend their endfeet and make contact with the pericyte-covered brain microvasculature, promoting tight junction formation [10-12]. The orchestrated interaction among these cells promotes BBB function. Additionally, pericytes and astrocytes provide homeostatic resilience to brain-insulting stimuli, such as stroke and neuroinflammation [13].
Damage to the BBB is known to be closely associated with neuropathology (Fig. 1) [14]. In Alzheimer’s disease (AD), BBB damage is observed with deposited amyloid-beta (Aβ) plaques around the brain microvasculature [15]. Although whether BBB damage precedes neuronal cell death in AD pathology remains controversial, some evidence suggests the possibility of BBB breakdown before neurodegeneration in the hippocampus [16-18]. Furthermore, metabolic syndrome is known to induce dementia. Among the dementia subtypes, vascular dementia plays a significant role in diabetes mellitus-associated dementia [19], suggesting that the BBB is disrupted by dysregulated metabolism. Furthermore, upon exposure to particulate matter due to air pollution, BBB damage has been observed [20-22]. Considering these clinical signatures, changes in the BBB in pathological conditions must be monitored and investigated to understand the underlying mechanism.
Over the last few decades, animal and Transwell models have been extensively utilized as alternative human BBB models [23,24]. Animal models allow the investigation of in vivo cell behaviors as a result of inter-organ interactions, whole-body circulation, and tissue-immune interactions, and are therefore considered to be suitable models for human substitutes [25]. Despite this capability, animal models have inherent limitations, such as genetic differences with humans, and thus cannot reflect human-specific responses in some cases [26-28]. Furthermore, end-point assays are generally used in animal models owing to the inability of real-time observations. In Transwell models, brain endothelial cells and pericytes or astrocytes can be attached on either side of a porous membrane, and thus, cells can be physically separated [29]. Due to their physical separation, individual cell types can be analyzed after cultivation. However, the Transwell system does not allow the replication of physiological conditions, such as tissue stiffness, a three-dimensional (3D) environment, and fluid flow. Engineered BBB models have arisen to resolve the unmet issues of animal and Transwell models [30].
Here, we present an overview of recent advances in engineered 3D BBB models. To this end, the key factors for the reconstitution of the BBB are discussed. The fabrication methods for 3D BBB structures are then presented. The application of 3D BBB models in the investigation of neuropathology is summarized, with a particular focus on the mimetics of pathological features. Finally, we provide perspectives for future research, arguing that 3D BBB models must be pursued as human-relevant alternative testing models.
The following issues must be overcome to resolve the limitations of animal models and two-dimensional (2D) Transwell models and faithfully mimic the in vivo human BBB physiology and pathology (Fig. 2).
First, the utilization of human-originated cells can resolve the issue of genetic heterogeneity (Fig. 2A). As discussed in many reports, clinical trials of drug candidates that show promising therapeutic effects in animal models are frequently discontinued to the lack of or limited efficacy in humans [31]. In some cases, human-gene knock-in mouse models did not fully recapitulate human disease pathologies [32]. Furthermore, some drugs (eg, thalidomide) showed no side effects in animal models, but had significant side effects when administered in humans [33]. These results show that animal models have limitations in modeling human physiology and pathology. The utilization of animal-originated cells in the fabrication of 3D in vitro models is problematic. Recently, Jang et al. [34] demonstrated that a 3D kidney model composed of animal cells showed different drug responses and fibrosis signatures from those obtained from human cell-based 3D kidney models. These results support the necessity of human-originated cells in modeling the human BBB.
Second, genetic information is needed to recapitulate genetic diseases (Fig. 2B). Some BBB-associated neurological diseases, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) or familial AD, originate from genetic mutations or defects [35,36]. To mimic genetic mutation-associated neuropathologies in a 3D BBB model, various patient-derived cells or induced pluripotent stem cells (iPSCs) with genetic mutations can be used. Transfection with disease-associated genes is a widely used technique. ReN cells are one of the successfully commercialized genetically modified neuronal progenitor cell lines that can differentiate into neurons and glial cells [37]. ReN cells have mutations in amyloid precursor protein (APP) and presenilin-1 (PSEN1), and these mutations induce AD phenotypes, such as deposition of amyloid plaques and tau tangles [38]. iPSCs constitute an excellent cellular model that reflects patients’ genetic information [39]. Since genetic information is maintained during the induction of iPSCs and subsequent differentiation processes, iPSC-derived cells can be used to model patient-specific disease phenotypes.
Third, the establishment of a physiologically relevant cell culture environment is important (Fig. 2C). Previous studies have indicated that cells cultured in a 2D environment showed different behaviors compared to those cultured in a 3D environment or residing in the in vivo tissue, in terms of migration, morphology, and drug response [40,41]. Recent studies have indicated that cells cultured in a Transwell system show different drug responses compared to 3D-cultured cells [42]. Considering that the Transwell system has a different microenvironment from in vivo tissue, it is desirable to mimic the 3D in vivo tissue environment in an engineered platform. Environmental factors include 3D hydrogel-based architecture, tissue-compatible stiffness, viscoelastic properties, interstitial flow, and tight cell-cell interactions [43-45]. The environment, including those factors, can be reconstructed using various methods such as organoids and organ-on-a-chip technologies [46].
The fabrication methods of 3D BBB models can be largely classified into 2 categories: bottom-up and top-down methods. Bottom-up methods refer to BBB structures generated via self-organization, such as vascularized organoids or vasculogenesis of brain vascular structures within a 3D hydrogel matrix. Top-down methods control the cell position by attaching cells to geometry-defined membranes or attaching endothelial cells to pre-formed hydrogel surfaces.
Bottom-up methods generate brain vasculature by promoting self-organization behaviors in brain endothelial cells. In vascularized brain organoids, brain endothelial cells are mixed with stem cells in a cell aggregation step, thus permitting the brain endothelial cells to form a vascular network within the developing brain organoids [47]. Alternatively, the addition of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), can promote vasculogenesis within brain organoids during differentiation by stimulating endothelial cell differentiation from stem cells [48]. As a straightforward method, BBB spheroids can be fabricated by aggregating brain endothelial cells, pericytes, and astrocytes [49,50]. In the case of the 3D hydrogel-based model, hydrogel-embedded or hydrogel surface-attached endothelial cells spontaneously form vascular networks via angiogenesis- or vasculogenesis-like behaviors [51-53]. In hydrogel-based methods, the cell types and hydrogel content are generally tailored to induce the efficient formation of brain microvasculature. These bottom-up methods provide morphologically in vivo-like capillary networks within cell aggregates or hydrogels. However, the capillary networks are not perfusable, and fluidic conditions cannot be established in vascularized and bulk-hydrogel-based methods.
Top-down methods utilize pre-formed microstructures as a support or a frame for blood vessel structures. For example, porous membranes are widely used to form a planar BBB model—that is, the Transwell system [54,55]. By attaching brain endothelial cells on top of the porous membrane and attaching pericytes and astrocytes on the backside of the membrane, cross-sectional BBB structures can be obtained. Recently, these porous membranes have been incorporated into microfluidic devices that can provide flow-induced stimuli to cultured cells [42,56,57]. Instead of a porous membrane, microstructures with regular spacing can be used as supporting structures for vasculature [58]. In a few studies, the lower channel of the porous membrane was filled with pericytes and astrocyte-embedded hydrogels, thus presenting 3D-like structures [59,60]. The micropost-based hydrogel patterning is a simple yet robust method to fabricate a 3D BBB structure [61,62]. When a sol-state hydrogel was introduced into the micropost array-decorated microfluidic chip, the hydrogel could not penetrate the interstitial region between the micropost owing to the surface tension, and thus subsequently solidified in a micropost-anchored state [63]. By attaching brain endothelial cells at the sidewall of the gelated hydrogel and readily incorporating pericytes and astrocytes in the hydrogel, a 3D BBB structure is formed. As an alternative templating method, microneedles, wires, soluble materials, or viscous liquids can be used to form microchannels within hydrogels [64-66]. By removing the templates after gelation of hydrogels, identical channel structures remain, and the brain endothelial cells are attached to the luminal surface of hydrogels. The attached cells form vascular structures, forming a structure-designed vascular structure. These top-down methods produce shape-controlled vascular structures and are thus suitable for reproducible experiments. However, in some cases, the structures may differ from in vivo vascular structures. The features of both fabrication methods are summarized in Table 1 [42,47-53,56-62,64-66].
In this section, we discuss how 3D BBB platforms are used in the study of neuropathologies (Fig. 3) [47,51,59,62].
The BBB acts as a transport barrier for drugs and particles in the normal state. For this reason, extensive efforts are being made to penetrate the BBB by modifying drug structures or the surface chemistry of nanoparticles [67]. Since the transport of drugs and nanoparticles cannot be visualized in the animal brain, in vitro-based BBB models are suitable models for identifying whether drugs or particles can be transported to the brain tissue (Fig. 3A) [59].
Ahn et al. [59] showed that high-density lipoprotein (HDL)-mimetic nanoparticles, termed engineered HDL-mimetic nanoparticles with apolipoprotein A1 (eHNP-A1), are transported by transcytosis. Therefore, they fabricated double-microstructure microfluidic devices with a porous membrane for vascular patterning and a micropost array for patterning of pericyte- and astrocyte-embedded hydrogels. They found that selective blocking of the scavenger receptor class B type 1 (SR-B1) or lipid transport-1 (BLT-1) reduced the translocation of nanoparticles into the hydrogel region. They found that SR-B1 regulates the uptake of HDL-like nanoparticles by brain microvascular endothelial cells, while BLT-1 controls the translocation of nanoparticles into the perivascular region. These results collectively suggest that eHNP-A1 transport through the BBB is regulated by transcytosis.
BBB chips are also useful for evaluating drug-associated BBB disruption and estimating the amount of drugs to be transported to the brain region. Maoz et al. [56] fabricated interconnected brain chip models by linking the influx of BBB, brain, and efflux BBB chips. They showed that methamphetamine temporarily disrupted the BBB, with effects that could be restored below a dose of 4 mM. Furthermore, the concentration of methamphetamine through the disrupted BBB was comparable to the in vivo values. For example, the ~10% proportion of methamphetamine observed in the perivascular channel in the BBBinflux chip was similar to the 20% to 30% plasma brain dialysate ratio observed in rats, and the concentration of ~100 μM in the brain chip was comparable to the concentrations of 0.23 to 310 μM observed in the brain tissues of chronic abusers. Therefore, this study suggests that the amount of drugs transported into the brain tissue can be estimated if the drugs are capable of disrupting the BBB.
Age is the most critical factor in neurodegenerative diseases, and the number of patients with neurodegenerative diseases is increasing concomitantly with population aging [68]. Although global pharmaceutical companies have attempted to develop therapeutics for neurodegenerative diseases, such as AD, the candidates frequently fail in clinical trials, even though they show promising therapeutic effects in animal models. These discrepancies are largely attributed to genetic heterogeneity in humans. With the help of genetic modification techniques, 3D in vitro models have shown promising capabilities in the recapitulation of AD pathologies. BBB damage is frequently observed in patients with AD (Fig. 3B) [51].
ReN cells with APP and PSEN1 mutations are good cell models that can mimic AD pathology, which leads to the deposition of amyloid plaques and tauopathy [38,69]. Shin et al. [61] controlled the interaction of AD neuronal cells and the BBB by separating a ReN cell-culture region and a BBB region by using a blank channel, and those 2 regions were connected after the endothelial barrier matured. Such spatial segregation before maturation can prevent undesirable early-time degeneration of the BBB structure. AD neuronal cells induced AD-like features in the brain microvasculature, such as decreased junctional protein expression and increased permeability, matrix-metalloproteinase-2 expression, and reactive oxygen production. Importantly, Aβ peptide deposition was observed in the vascular endothelium. They also showed that the transport of thrombin through the disrupted BBB caused neuronal cell death, which is observed in the brains of patients with AD [70], and such detrimental effects can be reduced by etodolac drug treatment.
Engineered in vitro BBB models help identify genetic mutation-specific susceptibility to AD pathology, particularly for cerebral amyloid angiopathy (CAA). The APOE4 mutation of apolipoprotein E is believed to be closely related to the onset of AD and CAA [71-73]. Blanchard et al. [51] co-cultured iPSC-induced brain endothelial cells, pericyte-like mural cells, and astrocytes in a 3D hydrogel. They found that mural cells with the APOE4 mutation induced significant accumulation of amyloid plaques at the vascular wall, while those with the APOE3 mutation did not show amyloid accumulation. They also found that the calcineurin-nuclear factor of activated T cells (NFAT) signaling is the key regulatory pathway in APOE4-associated CAA, and demonstrated the possibility of APOE4-induced CAA by inhibiting calcineurin-NFAT signaling pathways both in vitro and in vivo. These results show that genetic mutation-associated BBB pathologies can be recapitulated in human cell-based BBB models.
BBB function is closely related to brain inflammation. Since the cellular composition can be controlled in engineered BBB models, the contributing roles of BBB-composing cells in the modulation of inflammation can be studied. Seo et al. [64] reconstituted a neurovascular unit structure by coculturing 7 types of brain tissue cells, including brain endothelial cells, astrocytes, pericytes, oligodendrocytes, neural stem cells, microglia, and neurons. When lipopolysaccharide (LPS) was introduced through the perfusable endothelium, the existence of supporting cells mitigated the LPS-induced neuroinflammation compared to LPS exposure in the endothelial cell-only case. Although the contribution of individual cells needs further study, these results show that neurovascular unit-composing cells may help maintain brain tissue homeostasis.
3D BBB models can be used to study the mechanisms of fungal infection (Fig. 3C) [62]. Meningoencephalitis is induced by infection with Cryptococcus neoformans and causes 180,000 deaths worldwide, particularly in immunocompromised individuals [74,75]. Although various hypotheses have been proposed to explain the brain-specific infection and BBB penetration of C. neoformans, the underlying mechanism could not be explained due to the inability of experimental observation. Kim et al. [62] found that C. neoformans forms biofilm-like cell clusters on the brain endothelium, and this neurotropism was induced by the brain-abundant inositol. Other types of fungi, such as Candida glabrata and Cryptococcus deuterogattii, showed no notable neurotropism in the BBB chip, confirming the fungus-type-dependent neurotropism observed in clinical cases. Furthermore, they also showed that the BBB penetration of C. neoformans occurred through transcytosis and that transcytosis-mediated BBB penetration was regulated by the mpr1Δ gene. The BBB chip showed promising capabilities in the real-time monitoring of infection and investigation of underlying genetic and environmental factors.
The effect of the microenvironment is also an important factor in BBB formation and maturation. However, control of the microenvironment is not possible in vivo. One of the important factors in vasculogenesis is hypoxic conditions [76-78]. Inspired by the hypoxic conditions in the developmental process, Park et al. [42] differentiated human iPSCs in 5% oxygen conditions, and the differentiated cells were transferred to a microfluidic chip with a porous membrane for the formation of the BBB structure. BBB-composing cells differentiated under hypoxic conditions showed enhanced BBB functionality in terms of transporter expression, transendothelial electrical resistance, and transcytosis capability. These results demonstrate the importance of hypoxic conditions in the development and function of the BBB.
Brain organoid technology has opened a new era in the study of neural development. Assembled stem cells, such as iPSCs and embryonic stem cells, proliferate and spontaneously differentiate into brain organoids with spatially distinct patterns within them. Since the introduction of cerebral organoids by Lancaster et al. [79], various region-specific brain organoids have become available, including hippocampal-choroid plexus, cerebellum, forebrain, midbrain, and hypothalamic organoids [80-86]. Although these brain organoids have shown promising capabilities in mimicking the structure and function of the developing brain, they lack a vascular structure and are thus limited in the formation of large organoids and drug screening.
Recently, Cakir et al. [47] proposed an innovative method for inducing vascular structure formation in developing human cortical organoids (Fig. 3D). They formed human cortical organoids by mixing the human embryonic stem cells expressing the transcription factor ETS variant 2 and non-modified human embryonic stem cells in an optimized ratio. As expected, self-organized vascular-like structures were formed within the developing brain organoids, and the vascular system had BBB-like functionalities, including tight junction expression, nutrition transporters, and transendothelial electrical resistance. Furthermore, the vascular structure promoted the functional maturation of brain organoids, suggesting the importance of BBB-like vascular structures in the development of brain tissue.
Vascularization can also be induced by exposing brain organoids to pro-angiogenic factors. Ham et al. [48] consistently exposed stem cell aggregates to VEGF, which is known to be a key pro-angiogenic factor, starting on the day after self-aggregation. Some fractions of the embryonic stem cell line H9 differentiated into vascular endothelial cells and formed vascular networks with distinctive vascular markers and showed no reduction in neuronal markers. Prolonged exposure to VEGF and Wnt7a also induced the emergence of pericyte-like cells, as evidenced by immunostaining with alpha-smooth muscle actin.
Despite the achievements of recent in vitro BBB models, issues in modeling the physiology and pathology of the BBB remain.
First, consideration of the immune system is required to understand the mechanisms of neuropathologies. Recent studies have indicated that microglia are closely related to the onset and progression of neuropathologies [87]. For example, activated microglia in patients with AD induce neuronal cell death by secreting reactive oxygen species and inflammatory cytokines. Exposure to particulate matter also deteriorates the condition of brain cells due to the activation of microglia by stimulating the secretion of reactive oxygen species and inflammatory cytokines. Accumulating evidence indicates that circulating monocytes can enter the brain parenchyma through the disrupted BBB and colonize the microglial niche under pathological conditions [88,89], suggesting the possibility of monocyte-associated progression of AD pathology [90]. Furthermore, the activation of microglia precedes the spread of tau pathology [91]. Since cellular interaction is believed to be a key factor in the progression of neuropathologies, consideration of the brain immune system is required.
Second, the circulation of cerebrospinal fluid in the brain tissue should be mimicked in an in vitro system. Recently, the existence of the glymphatic system [92,93] and brain lymphatic system [94,95] was reported. Although further in-depth studies are needed, these brain-circulating systems are believed to play roles in the clearance of metabolic wastes and neurotoxic proteins [96]. Considering that the BBB participates in the transport of neurotoxic proteins and brain metabolism, the simultaneous consideration of the BBB and these brain-circulating systems can reveal novel scientific mechanisms of neuropathologies.
Third, BBB models can be used as a test platform for nanomedicine. As shown in many examples, BBB models have the capability to be used to monitor the transport of nanomaterials. Nanomaterials have recently emerged as a powerful tool for delivering biochemical factors to the brain tissue due to their ease of surface functionalization and cargo control [97-98]. Since non-optimized nanomaterials are prone to be blocked by the BBB during transport, BBB models can assist in the development and optimization of nanomedicine vehicles suitable for penetrating the BBB.
Finally, the commercialization of BBB chips is also important for cost-effective and time-efficient drug development. Various types of organ-on-a-chip platforms are now available on the market, and global pharmaceutical companies are interested in their utilization in drug development [99,100]. Considering that the Transwell model presents a non-physiological tissue environment and animal models are very costly, the development of an alternative platform is necessary. Therefore, the commercialization of BBB models and their application in the drug development process can revolutionize the pharmaceutical industry. For commercialization, the design and materials for BBB chips need to be optimized for mass production and high-content screening.
In this review, we summarize recent advances in 3D BBB systems that show promising capabilities as human BBB models. These models have been prepared using human cells in many cases and successfully reflected the genetic information of human neuropathologies. Furthermore, 3D BBB models have also been used to model infection and inflammation and have demonstrated the effects of cell-cell or inter-organ interactions by coculturing multiple cell types or multi-organ compartments in engineered systems. Despite their promising capabilities, some issues remain, such as consideration of the immune and glymphatic systems. It is believed that 3D BBB models can be used to evaluate drug delivery and investigate human brain neuropathologies.
NOTES
Conflict of interest
Hong Nam Kim has been an editor of Organoid since 2021. No other potential conflict of interest relevant to this article was reported.
Fig. 1.
Graphical summary of a normal and disrupted blood-brain barrier (BBB). The BBB is composed of 3 types of cells, and the abnormal degeneration, activation, or genetic mutations of BBB-composing cells lead to disruption of the BBB. The disrupted BBB induces leakage of molecules through the permeable vascular wall due to the loss of tight junctions. Functional damage of the BBB is associated with various brain pathologies, such as neurodegenerative diseases and neuroinflammation.
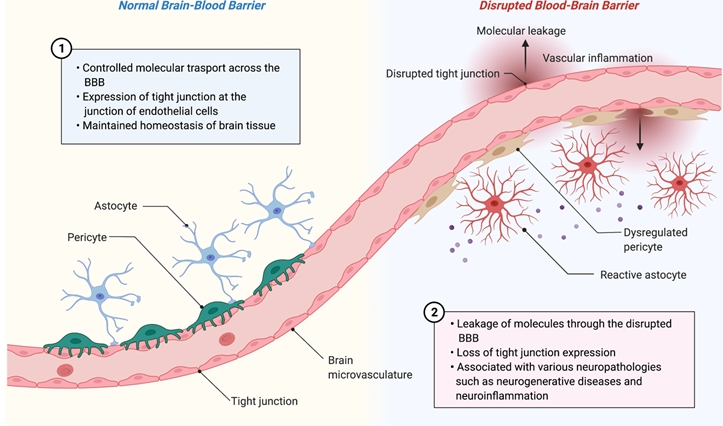
Fig. 2.
Key factors to consider for recapitulation of the human blood-brain barrier (BBB) physiology and pathology. (A) Utilization of human-originated cells for reconstructing in vitro BBB models. (B) Reflection of genetic mutation information in BBB-composing cells. (C) Consideration of microenvironmental factors, such as mechanical properties and the composition of hydrogels, fluidic conditions, molecular transport, and cell-cell interactions.
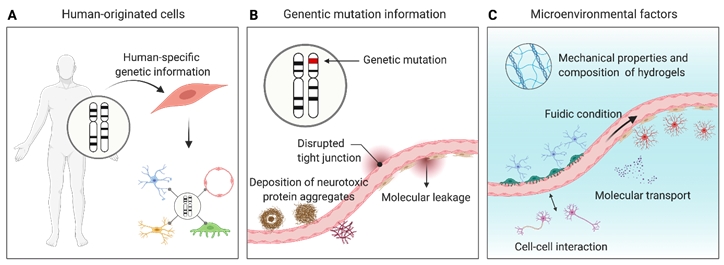
Fig. 3.
Utilization of in vitro blood-brain barrier (BBB) models in the study of various BBB-associated physiology and pathologies. (A) Particle and drug transport through the BBB. (A-i) Schematic illustration of particle and drug transport. (A-ii) Graphical illustration of a BBB chip for monitoring nanoparticle transport. (A-iii) Fluorescence images of nanoparticles (red) uptaken by brain microvascular endothelial cells (BMECs) and human astrocytes (HAs). Scale bar: 50 μm. Reproduced from Ahn et al. Nat Commun 2020;11:175, according to the Creative Commons license [59]. (B) Genetic mutation-associated neuropathology study. (B-i) Schematic illustration of genetic mutation-associated neuropathology. (B-ii) Graphical illustration of a 3D BBB model made of induced pluripotent stem cells (iPSC)-derived cells. (B-iii) Fluorescence images of accumulated non-vascular (red) and vascular amyloid (green). Scale bar: 10 μm. BECs, brain endothelial cells; iMCs, iPSC-derived mural cells. Reproduced from Blanchard et al. Nat Med 2020;26:952-63, with permission from Springer Nature [51]. (C) Brain infection and neuroinflammation study. (C-i) Schematic illustration of brain infection and neuroinflammation. (C-ii) Photograph of BBB chip for studying fungal infections. (C-iii) Microscopic images of fungus-type- and genetic-mutation-dependent colonization. Scale bar: 100 μm. Reproduced from Kim et al. Nat Biomed Eng 2021;5:830-46, with permission from Springer Nature [62]. (D) Development of BBB structures in the brain organoids. (D-i) Schematic illustration of vascularization in brain organoids. (D-ii) Microscopic images of cultured brain organoids without (human cortical organoid, hCO) or with vasculature (vascularized human cortical organoid, vhCO). Scale bar: 4 mm. (D-iii) Immunofluorescence images of vasculature in hCO and vhCO. Scale bar: 100 μm. Reproduced from Cakir et al. Nat Methods 2019;16:1169-75, with permission from Springer Nature [47]. CFW, calcofluor white; WT, wild-type.
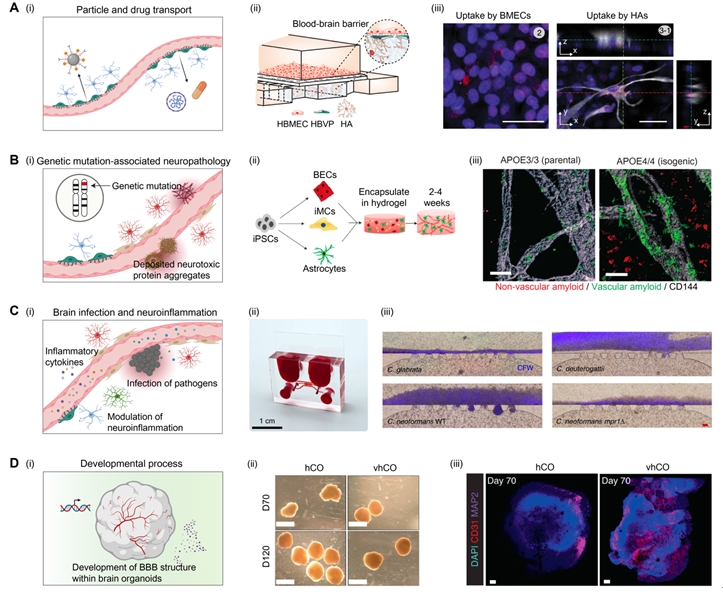
Table 1.
Fabrication methods for three-dimensional (3D) blood-brain barrier (BBB) models
| Classification | Fabrication principle | Features | References |
|---|---|---|---|
| Bottom-up | Vascularization of organoids or spheroids | - Self-organized vascular structure within the brain organoids | [47-50] |
| - Induced by exposing stem cells to pro-angiogenic factors or genetic modification for endothelial cell differentiation | |||
| - Capillary-like morphology with BBB-associated marker expression | |||
| - Commonly floating in the culture medium | |||
| - Non-perfusable vascular structure | |||
| Embedding in a hydrogel | - Self-organized vascular structure within the hydrogels | [51-53] | |
| - Induced by mixing brain endothelial cells, pericytes, and astrocytes in the hydrogel and letting them self-organize | |||
| - Capillary-like morphology with BBB-associated marker expression | |||
| - Non-perfusable vascular network | |||
| Top-down | Attachment of BBB-composing cells on a porous membrane or on micropatterns that are incorporated in a microfluidic chip | - Attaching brain endothelial cells on top of a porous membrane and pericytes/astrocytes on the opposite side of the porous membrane | [42,56-58] |
| - Planar BBB structure with BBB-associated marker expression | |||
| - Interaction of cells through the pores | |||
| - Fluidic stimulation is available | |||
| - Transendothelial electrical resistance can be measured | |||
| - Two-dimensional structure | |||
| Attachment of endothelial cells on a porous membrane and embedding pericytes and astrocytes in the underlying 3D matrix | - Attachment of endothelial cells on a porous membrane and embedding pericytes and astrocytes in the underlying 3D matrix | [59,60] | |
| - Pericytes and astrocytes are cultured in a 3D environment | |||
| - Fluidic stimulation is available | |||
| - Transport into hydrogel can be monitored | |||
| Micropost array-assisted hydrogel patterning | - Encasing of cell-laden hydrogel in a defined shape by using micropost array as guidance for hydrogel filling | [61,62] | |
| - Pericyte- and astrocyte-laden 3D hydrogel in contact with hydrogel-surface covered vascular wall | |||
| - Vascular sprouting, such as angiogenesis, can be recapitulated | |||
| - Interstitial flow and luminal flow are available | |||
| - Quasi-3D structure | |||
| Removal of templates (microneedles, wires, soluble materials) | - Removal of the template after hydrogel gelation for the formation of the microchannel in which brain endothelial cells are cultured | [64-66] | |
| - Pericyte and astrocytes are embedded in the hydrogel before gelation | |||
| - Interstitial flow and luminal flow are available | |||
| - 3D structure | |||
| - The size and shape of the microvasculature are predefined |
References
1. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010;37:13-25.


2. Bagchi S, Chhibber T, Lahooti B, Verma A, Borse V, Jayant RD. In-vitro blood-brain barrier models for drug screening and permeation studies: an overview. Drug Des Devel Ther 2019;13:3591-605.



3. Seo S, Kim H, Sung JH, Choi N, Lee K, Kim HN. Microphysiological systems for recapitulating physiology and function of blood-brain barrier. Biomaterials 2020;232:119732.


4. Barar J, Rafi MA, Pourseif MM, Omidi Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts 2016;6:225-48.




5. Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res 2007;24:1759-71.




6. Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 2009;1788:842-57.


7. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004;16:1-13.


8. Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature 2010;468:557-61.



9. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010;468:562-6.




10. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006;7:41-53.



11. Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia 2013;61:1939-58.



12. Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987;325:253-7.



13. Webb AA, Muir GD. The blood-brain barrier and its role in inflammation. J Vet Intern Med 2000;14:399-411.


14. Sivandzade F, Cucullo L. In-vitro blood-brain barrier modeling: a review of modern and fast-advancing technologies. J Cereb Blood Flow Metab 2018;38:1667-81.



15. Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 2017;107:41-56.



16. Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015;85:296-302.



17. Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 2012;11:868-77.



18. Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging 2010;31:1077-88.


19. Alsharif AA, Wei L, Ma T, et al. Prevalence and incidence of dementia in people with diabetes mellitus. J Alzheimers Dis 2020;75:607-15.


20. Shou Y, Huang Y, Zhu X, Liu C, Hu Y, Wang H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol Environ Saf 2019;174:344-52.


21. Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 2008;36:289-310.


22. Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Münzel T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci 2020;21:4306.



23. Wolff A, Antfolk M, Brodin B, Tenje M. In vitro blood-brain barrier models: an overview of established models and new microfluidic approaches. J Pharm Sci 2015;104:2727-46.


24. Coisne C, Dehouck L, Faveeuw C, et al. Mouse syngenic in vitro blood-brain barrier model: a new tool to examine inflammatory events in cerebral endothelium. Lab Invest 2005;85:734-46.



25. Banks WA. Mouse models of neurological disorders: a view from the blood-brain barrier. Biochim Biophys Acta 2010;1802:881-8.


26. Yin Z, Raj D, Saiepour N, et al. Immune hyperreactivity of Aβ plaque-associated microglia in Alzheimer’s disease. Neurobiol Aging 2017;55:115-22.


27. Galatro TF, Holtman IR, Lerario AM, et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci 2017;20:1162-71.



28. Friedman BA, Srinivasan K, Ayalon G, et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep 2018;22:832-47.


29. Jamieson JJ, Searson PC, Gerecht S. Engineering the human blood-brain barrier in vitro. J Biol Eng 2017;11:37.




30. Oddo A, Peng B, Tong Z, et al. Advances in microfluidic blood-brain barrier (BBB) models. Trends Biotechnol 2019;37:1295-314.


31. Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114-8.


32. Sun J, Zhuang Z, Zheng J, et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 2020;182:734-43.



33. Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci 2011;122:1-6.



34. Jang KJ, Otieno MA, Ronxhi J, et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med 2019;11:eaax5516.


35. Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol 2015;78:887-900.


36. Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 2010;23:213-27.



37. Park J, Wetzel I, Marriott I, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci 2018;21:941-51.




38. Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014;515:274-8.




39. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 2020;21:571-84.




40. Duval K, Grover H, Han LH, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 2017;32:266-77.



41. Kapałczyńska M, Kolenda T, Przybyła W, et al. 2D and 3D cell cultures: a comparison of different types of cancer cell cultures. Arch Med Sci 2018;14:910-19.


42. Park TE, Mustafaoglu N, Herland A, et al. Hypoxia-enhanced blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 2019;10:2621.




43. Kim HN, Choi N. Consideration of the mechanical properties of hydrogels for brain tissue engineering and brain-on-a-chip. BioChip J 2019;13:8-19.


44. Jang KJ, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb) 2013;5:1119-29.



45. Jo Y, Choi N, Kim K, Koo HJ, Choi J, Kim HN. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 2018;8:5259-75.



46. Bang S, Lee S, Choi N, Kim HN. Emerging brain-pathophysiology-mimetic platforms for studying neurodegenerative diseases: brain organoids and brains-on-a-chip. Adv Healthc Mater 2021;10:e2002119.


47. Cakir B, Xiang Y, Tanaka Y, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods 2019;16:1169-75.




48. Ham O, Jin YB, Kim J, Lee MO. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun 2020;521:84-90.


49. Cho CF, Wolfe JM, Fadzen CM, et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun 2017;8:15623.




50. Bergmann S, Lawler SE, Qu Y, et al. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc 2018;13:2827-43.




51. Blanchard JW, Bula M, Davila-Velderrain J, et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med 2020;26:952-63.




52. Lee S, Chung M, Lee SR, Jeon NL. 3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro. Biotechnol Bioeng 2020;117:748-62.


53. Bang S, Lee SR, Ko J, et al. A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci Rep 2017;7:8083.




54. Cecchelli R, Aday S, Sevin E, et al. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One 2014;9:e99733.



55. Di Marco A, Vignone D, Gonzalez Paz O, et al. Establishment of an in vitro human blood-brain barrier model derived from induced pluripotent stem cells and comparison to a porcine cell-based system. Cells 2020;9:994.



56. Maoz BM, Herland A, FitzGerald EA, et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat Biotechnol 2018;36:865-74.




57. Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012;12:1784-92.


58. Deosarkar SP, Prabhakarpandian B, Wang B, Sheffield JB, Krynska B, Kiani MF. A novel dynamic neonatal blood-brain barrier on a chip. PLoS One 2015;10:e0142725.



59. Ahn SI, Sei YJ, Park HJ, et al. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat Commun 2020;11:175.




60. Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015;9:054124.



61. Shin Y, Choi SH, Kim E, et al. Blood-brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv Sci (Weinh) 2019;6:1900962.



62. Kim J, Lee KT, Lee JS, et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier. Nat Biomed Eng 2021;5:830-46.



63. Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013;13:1489-500.


64. Seo S, Choi CH, Yi KS, et al. An engineered neurovascular unit for modeling neuroinflammation. Biofabrication 2021;May 5 13:035039.

65. Herland A, van der Meer AD, FitzGerald EA, Park TE, Sleeboom JJ, Ingber DE. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS One 2016;11:e0150360.



66. Yu F, Kumar ND, Foo LC, Ng SH, Hunziker W, Choudhury D. A pump-free tricellular blood-brain barrier on-a-chip model to understand barrier property and evaluate drug response. Biotechnol Bioeng 2020;117:1127-36.


67. Hajal C, Campisi M, Mattu C, Chiono V, Kamm RD. In vitro models of molecular and nano-particle transport across the blood-brain barrier. Biomicrofluidics 2018;12:042213.



69. Kim YH, Choi SH, D’Avanzo C, et al. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat Protoc 2015;10:985-1006.




70. Grammas P, Martinez JM. Targeting thrombin: an inflammatory neurotoxin in Alzheimer’s disease. J Alzheimers Dis 2014;42 Suppl 4:S537-44.


71. Ringman JM, Sachs MC, Zhou Y, Monsell SE, Saver JL, Vinters HV. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol 2014;71:878-83.



72. Greenberg SM, Briggs ME, Hyman BT, et al. Apolipoprotein E epsilon 4 is associated with the presence and earlier onset of hemorrhage in cerebral amyloid angiopathy. Stroke 1996;27:1333-7.


73. Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol 1995;38:254-9.


74. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017;17:873-81.



75. May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 2016;14:106-17.



76. Kusuma S, Peijnenburg E, Patel P, Gerecht S. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler Thromb Vasc Biol 2014;34:913-20.



77. Lee SW, Jeong HK, Lee JY, et al. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med 2012;4:924-38.



78. Han Y, Kuang SZ, Gomer A, Ramirez-Bergeron DL. Hypoxia influences the vascular expansion and differentiation of embryonic stem cell cultures through the temporal expression of vascular endothelial growth factor receptors in an ARNT-dependent manner. Stem Cells 2010;28:799-809.



79. Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373-9.



80. Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 2015;6:8896.



81. Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 2015;10:537-50.


82. Paşca AM, Sloan SA, Clarke LE, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015;12:671-8.




83. Jo J, Xiao Y, Sun AX, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 2016;19:248-57.



84. Xiang Y, Tanaka Y, Patterson B, et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 2017;21:383-98.



85. Monzel AS, Smits LM, Hemmer K, et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Reports 2017;8:1144-54.



86. Krefft O, Jabali A, Iefremova V, Koch P, Ladewig J. Generation of standardized and reproducible forebrain-type cerebral organoids from human induced pluripotent stem cells. J Vis Exp 2018;131:56768.

87. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 2021;17:157-72.



88. Bruttger J, Karram K, Wörtge S, et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 2015;43:92-106.


89. Priller J, Flügel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 2001;7:1356-61.



90. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018;14:133-50.




91. Streit WJ, Braak H, Del Tredici K, et al. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 2018;66:2550-62.


92. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111.



93. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 2018;13:379-94.



94. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-41.




95. Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018;560:185-191.




96. Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 2018;100:375-88.



97. Jena L, McErlean E, McCarthy H. Delivery across the blood-brain barrier: nanomedicine for glioblastoma multiforme. Drug Deliv Transl Res 2020;10:304-18.



98. Tang W, Fan W, Lau J, Deng L, Shen Z, Chen X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev 2019;48:2967-3014.


- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 4,710 View
- 42 Download
- ORCID iDs
-
Hong Nam Kim

https://orcid.org/0000-0002-0329-0029 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print