Recent advances in multicellular human liver models
Article information
Abstract
The liver is the most important metabolic organ in the body. Model systems that recapitulate the complex organ structure and cell composition of the human liver are insufficient to study liver biology and to test toxicity and efficacy during new drug development. Recently established 3-dimensional liver models, including spheroids and organoids, organs-on-a-chip, bioprinting, and the decellularization/recellularization technique, have provided platforms that emulate the structural and functional characteristics of the human liver better than conventional 2-dimensional cell culture models and animal models. This review summarizes the architecture and cell compositions of human liver tissue, focusing on recent studies of multicellular human liver models that recapitulate in vivo-like physiologies with morphological and functional advances by the cellular communication of parenchymal and non-parenchymal cells. We discuss the applications, limitations, and future perspectives of advanced multicellular human liver models.
Introduction
The liver is a central organ in various metabolic processes, and its primary functions are (1) metabolism of carbohydrates, lipids, amino acids, and bile acids; (2) detoxification; (3) synthesis of plasma proteins such as albumin and clotting factors; and (4) storage of glycogen, vitamins, and minerals [1]. Hepatic failure arises due to the deterioration of liver function, mainly owing to xenobiotics or disease progression, and is often fatal. Unfortunately, reliable liver models for predicting the risk of hepatotoxicity during new drug development and evaluating the efficacies of drugs to target liver diseases are insufficient.
Three-dimensional (3D) liver models, including spheroids and organoids, have recently been developed, and their advantages were highlighted with respect to recapitulation of the complexities of cell compositions and tissue structure in comparison with 2-dimensional (2D) cell culture models. Primary human hepatocytes (PHHs) isolated from human liver tissue have high functionality and are therefore generally used as the gold standard for an in vitro drug testing platform. However, their viability and functionality are not sufficiently maintained in conventional 2D culture formats. Approaches to manage the 3D microenvironment by controlling stiffness using extracellular matrix (ECM), such as Matrigel and hydrogel, have been implemented and improved the viability of PHHs until 40 days in culture [2,3]. In addition, interactions between hepatocytes and non-parenchymal cells, such as endothelial cells and hepatic stellate cells (HSCs), in 3D hepatic spheroids improved viability and the liver phenotype [4,5]. Importantly, ameliorated liver functions, including high cytochrome P450 (CYP) enzyme activities, improved toxicity prediction in these 3D liver models.
As an alternative source of hepatic cells, protocols have been developed to differentiate induced pluripotent stem cells (iPSCs) into liver tissue cells, including hepatocytes [6], HSCs [7], and Kupffer cells (KCs) [8]. Furthermore, organoid generation protocols have been developed by using a 2D differentiation protocol in combination with 3D culture systems to provide a tissue microenvironment. Liver organoids have been generated from adult human liver tissue and remain stable and functional for a long time [9]. However, tissue-derived organoids mainly contain parenchymal cell types of liver tissue, such as hepatocytes and cholangiocytes, under epithelial cell type-enriched culture conditions. Therefore, iPSC-derived hepatic endoderm cells were co-cultured with endothelial cells and mesenchymal stem cells (MSCs) [10]. Furthermore, iPSC-based liver organoids containing multi-lineage cells were developed depending on their pluripotency [11]. This review summarizes the structures and cell types of human liver tissue and in vivo-mimicking liver models, specifically focusing on the recent advances and future applications of multicellular human liver models with potential use for investigating liver diseases and testing promising therapeutic drugs.
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Liver architecture and cell composition
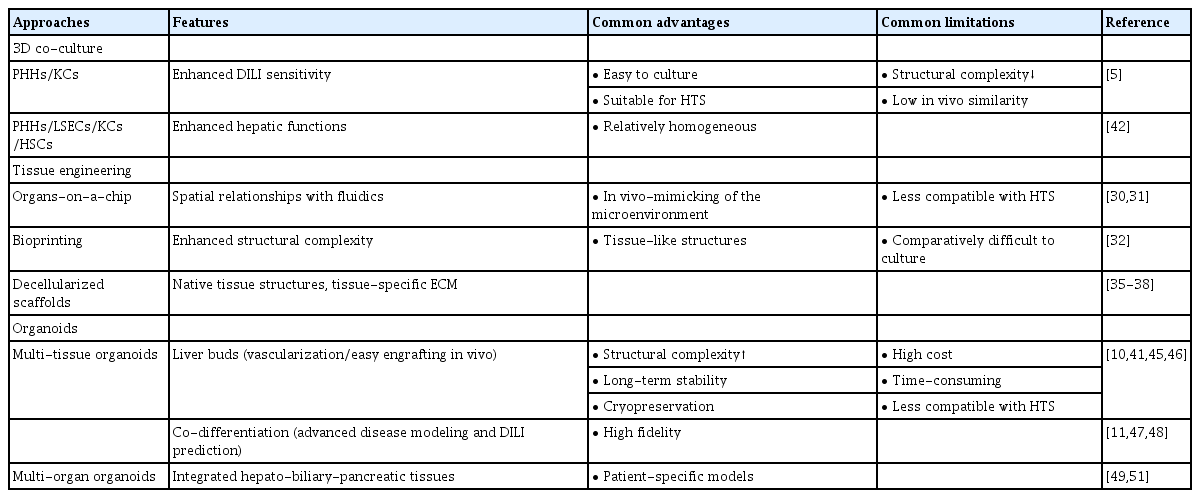
The basic architectural unit of the liver is the hepatic lobule, which consists of hepatocyte plates lined by sinusoidal capillaries radiating toward the central vein (Fig. 1A). Hepatic lobules are nearly hexagonal and each is delimited by the portal triad, consisting of the portal vein, hepatic artery, and bile duct. The portal vein and hepatic artery provide blood to the lobules through a sinusoidal capillary network from the portal triad to the central vein, while bile synthesized by hepatocytes flows in the opposite direction to the bile duct. Hepatocytes are the main parenchymal cell type of the liver and account for approximately 70% of liver cells and most of the liver volume [12]. Cholangiocytes and non-parenchymal stromal cells, including liver sinusoidal endothelial cells (LSECs), HSCs, and KCs are also important components of the liver (Fig. 1B and Fig. 2) [13–28].
Liver architecture and cell composition. (A) Structural organization of the liver, (B) Cell localization in the hepatic lobule, (C) Apicobasal membrane polarity in hepatocytes. LSEC, liver sinusoidal endothelial cell; HSC, hepatic stellate cell; KC, Kupffer cell.

Cell types of the liver and their major functions and representative markers. ALB, albumin; ACTA2: actin alpha 2, smooth muscle; HNF4A, hepatocyte nuclear factor 4 alpha; KRT, keratin; SOX9, SRY-Box transcription factor 9; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; STAB2, stabilin 2; ID I/III, inhibitor of DNA binding 1/3; CLEC-4F, C-type lectin domain family 4 member F; COL1A1, collagen type I alpha; PDGFRβ, platelet-derived growth factor receptor beta.
Hepatocytes are positioned with their basolateral surface facing perforated LSECs, which facilitates the delivery of endocrine secretions into the bloodstream (Fig. 1C). Bile acids and bile salts are transported across the bile canalicular-apical surface of hepatocytes, and the basolateral and apical membranes are separated by tight junctions between adjacent hepatocytes [29]. The arrangement between polarized hepatocytes and capillaries and bile duct cells is associated with the endocrine and exocrine functions of the liver [30].
Cholangiocytes are the other parenchymal cells in the liver. They are highly specialized epithelial cells that line the biliary tree, a network of bile ducts, and account for approximately 3% to 5% of liver cells [22,28]. The major physiological functions of cholangiocytes are bile secretion, modification, and homeostasis through transport of various ions and water in a complex liver anatomic niche [31]. They also play a central role in liver regeneration in pathologic conditions [32].
LSECs are specialized hepatic endothelial cells that line the hepatic sinusoids, a capillary network in the liver, and are the most abundant non-parenchymal cells in the liver, accounting for approximately 15% to 20% of liver cells [33,34]. They are one of the most permeable endothelial barriers, allowing an easy exchange of metabolites and maintenance of homeostasis within the liver [16]. LSECs are also a selective barrier positioned at the interface between the oxygen- and nutrient-rich blood side and abluminal side with hepatocytes and HSCs; they are crucial for nutrient exchange, active uptake, the metabolism of proteins, lipids, and glucose, and the clearance of waste biomolecules and xenobiotics including drugs [34].
KCs are tissue-resident macrophages in the liver sinusoids and are the first line of immune cells that respond to infectious materials transported from the gastrointestinal tract via the portal vein [35]. Their major functions are involved in host systemic defense under physiologic conditions and in disease progression and resolution under pathologic conditions through phagocytosis, immunomodulation, and metabolic functions [36]. Liver injuries that overwhelm the gatekeeping function of KCs result in inflammation, fibrosis, and eventually hepatocellular carcinoma; therefore, KCs are important target cells for the treatment of liver diseases [37].
HSCs are positioned in the space of Disse, the perisinusoidal space between a hepatocyte and sinusoid (Fig. 1B). They mainly store vitamin A in a quiescent state and play important roles in regulating blood flow and maintaining the ECM balance by acting as pericyte-like cells in the liver [38]. Various insults that cause liver injury activate HSCs to transdifferentiate into myofibroblast-like cells, leading to collagen accumulation and fibrosis [39,40].
In vivo-mimicking liver models
1. 3D co-culture
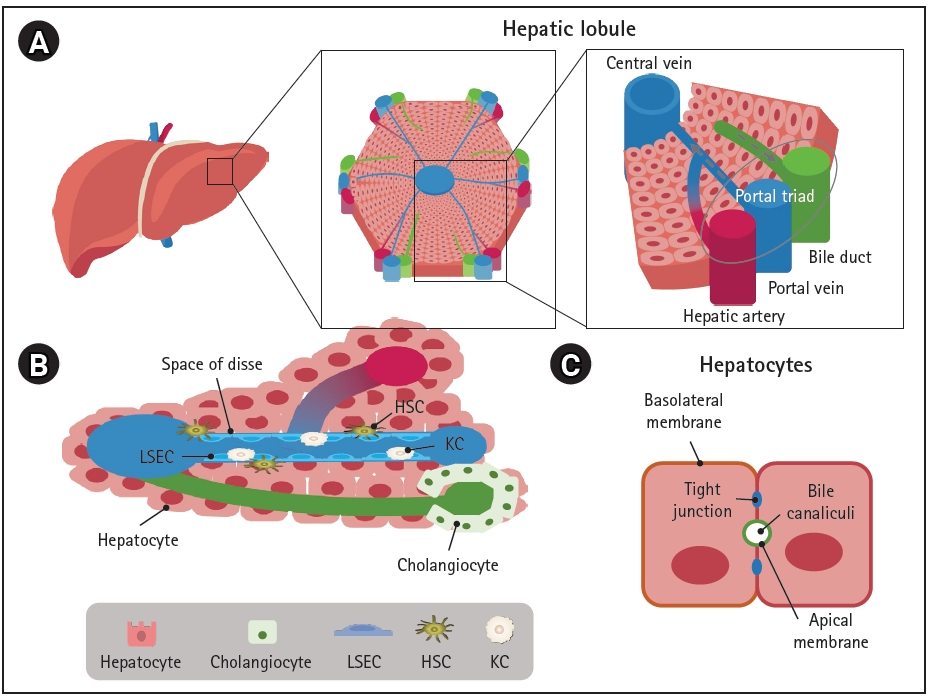
There is no perfect in vivo model of the liver; however, several in vitro multi-lineage liver models have been extensively developed (Fig. 3 and Table 1) [5,10,11,30–32,35–38,41–55]. Spheroids, which are 3D cultures of hepatic parenchymal and non-parenchymal cells, are among the most commonly used liver models [56] (Fig. 3A). Spheroids consisting of PHHs and non-parenchymal cells demonstrated improved metabolic function and stability in culture; thus, long-term drug treatment could be evaluated for drug-induced liver injury (DILI) [5]. Importantly, the sensitivity of 3D hepatic spheroids to known hepatotoxicants and their predictive value were better than those of plated 2D PHHs [5]. Moreover, spheroids are relatively homogeneous, easy to culture, and cost-effective; thus, they may be a more suitable model for high-throughput screening (HTS) than the more complex model systems described next. However, they show lower structural complexity and poorer in vivo fidelity than native tissue, which remain as limitations.
In vivo-mimicking multicellular human liver models and their applications. (A) tissue engineering, (C) organoids, (D) applications.
2. Tissue engineering
While spheroids primarily emulate 3D cell-cell interactions, tissue engineering technologies can provide the surrounding microenvironment to recapitulate the microarchitecture of native tissue and mechanical properties such as blood flow. Organs-on-a-chip system can simulate the positional specificity of each cell type and their spatial relationships (Fig. 3B). Polarized hepatocytes separated by a lining of endothelial cells to mimic the space of Disse generated using fluidics exhibited long-term survival and increased CYP enzyme activities [50,55]. Bioprinting technology can also provide micro-architectural properties using bioink-containing cells, growth factors, and biomaterials (Fig. 3B). Bioprinted liver models inspired by the liver lobular structure have been constructed. A liver ECM-based bioink has been designed to encapsulate liver cells and thereby mimic natural tissue properties, and cords of hepatocytes with a functional sinusoidal lumen-like network in both horizontal and vertical orientations were designed. The bioprinted liver model demonstrated enhanced albumin production, urea synthesis, and CYP enzyme activities [46].
An important element of 3D modeling is the types and concentrations of ECM molecules, which are essential for cell attachment, proliferation, and differentiation, and are mainly proteins, including collagens, elastin, and fibronectin, polysaccharides, and proteoglycans [57,58]. Decellularization of tissues or organs is a good method to prepare ECM that potentially maintains the natural microenvironment and architecture of the tissue [44,47] (Fig. 3B). Recellularized rat liver successfully preserved the survival and function of hepatocytes in vitro, and, upon transplantation of recellularized liver grafts into rats, the micro-anatomical organ structure, including the vascular network and biliary tract, was maintained and the function was similar to that of the adult liver [54]. Moreover, decellularized ECM can improve the hepatic maturation of stem cells. Bone marrow-derived MSCs cultured in a decellularized liver scaffold as spheroids exhibited mature and stable metabolic functions compared with those cultured in a 2D system. Notably, liver-specific ECM provides an improved environment for hepatic differentiation and maturation of stem cells [41].
3. Organoids
Organoids are stem cell-based self-organized 3D models that recapitulate the cell composition and physiological niche of the corresponding tissue [59]. Liver organoids can be generated from adult tissue stem cells [9,60] and pluripotent stem cells (PSCs) [53,61]. While tissue-derived organoids are generated in a relatively short amount of time and are genetically stable even after long-term passage [9], they have limited characteristics and differentiation potential owing to the characteristics of the cell source [62]. Meanwhile, PSCs, including embryonic stem cells and iPSCs, can differentiate into the three germ layers (ectoderm, mesoderm, and endoderm), which can develop into all types of cells and tissues. Therefore, PSCs can generate multi-lineage organoids from a single donor.
Multi-tissue organoids composed of cells of multiple germ layers within a single organ can be generated by intra-organ self-organization [63]. Multi-organ organoids can be generated by inter-organ self-organization through interconnectivity between organ domains [63] (Fig. 3C). Multi-tissue “liver bud” organoids have been generated by co-culturing cells derived from different germ layers, such as hepatic endoderm cells derived from iPSCs, human umbilical vein endothelial cells, and MSCs [10]. Liver buds composed of cells derived entirely from iPSCs were further developed [52]. Multi-tissue organoids can be used to examine multi-lineage communication and were used to reveal that vascular endothelial growth factor signaling crosstalk between hepatoblasts and endothelial cells strengthens endothelial network formation and hepatic differentiation [43]. When liver buds were transplanted into the kidney capsule or cranial window in vivo, they connected to the host vessel within 48 hours and matured to show functionality similar to that of the adult liver [43,53]. Furthermore, co-differentiation methods have been reported [11,45,51], and liver organoids consisting of hepatocyte-like cells, biliary cells, HSCs, and KCs were generated from iPSCs [11]. Single-cell RNA sequencing revealed that the model contained about 59.2% hepatocyte-like cells, 31% stellate-like cells, 8.9% biliary-like cells, and 0.8% Kupffer-like cells. Steatohepatitis has been successfully modeled with oleic acid (OA) treatment, and progressive disease phenotypes, such as increased lipid accumulation, triglyceride storage, inflammatory signaling, and stiffness, which represent the severity of fibrosis, were observed in OA-treated multicellular liver organoids [11]. In addition, high-fidelity DILI prediction was possible by evaluating cholestatic and/or mitochondrial toxicity using established organoids [51]. Another multi-lineage organoid model was developed for liver fibrosis, and these organoids have a ductal and vascular structure and contain mesenchymal cells [45]. Congenital hepatic fibrosis has been successfully modeled by engineering the causative mutation into organoids, and collagen-producing myofibroblasts, which are essential mediators of liver fibrosis, and the platelet-derived growth factor receptor beta (PDGFRB)-STAT3 pathway were activated. To this end, treatment with PDGFRB inhibitors elicited an anti-fibrotic effect in the organoid model of liver fibrosis [45]. These multi-tissue organoids faithfully emulate complex features of disease progression and can be used to evaluate toxicity and develop novel therapies. Finally, multi-organ organoids containing hepato-biliary-pancreatic organ domains have been established, followed by human endoderm organogenesis [48,64]. Hepato-biliary-pancreatic tissues developed at the boundary between anterior and posterior gut spheroids generated by iPSCs [48]. Retinoic acid signaling from non-epithelial cells in the anterior region at the foregut-midgut boundary was important for lineage specification [49]. Structurally and functionally integrated multi-organ organoids can be valuable models for performing mechanistic studies of complex organogenesis specifically in humans, modeling congenital disorders and related diseases, and developing therapeutic applications.
Applications, limitations, and future perspectives
Multicellular liver models including spheroids, organoids, and various 3D model systems in combination with tissue engineering technologies are useful alternatives to traditional in vitro and animal models. They have been used for diverse applications from basic research to therapeutic development, including modeling of human development and diseases, drug screening, toxicity testing, and regenerative medicine (Fig. 3D). Multicellular systems can model spatiotemporal cell-cell and cell-ECM communications because they recapitulate the structural and functional complexity of the liver tissue, which is difficult to achieve with 2D models. Notably, organoids can be generated using iPSCs or patient-derived cells, which may provide patient-specific models for the evaluation of differences in patients’ responses to personalized medicine. However, 3D modeling is time-consuming, labor-intensive, and less compatible with HTS, and these disadvantages are amplified according to the complexity of the physiological niche being mimicked [52,65]. An automatic platform has been adopted for robust and consistent generation, culture, and drug testing of organoids. Quality control and reproducibility are challenging owing to the heterogeneity of the models, and higher resolution techniques such as single-cell RNA sequencing and multi-omics are required to characterize multicellular systems. Chemically defined ECM and cost-efficient processes for large-scale manufacturing remain to be developed. The recent advances in multicellular human liver models may provide promising systems for biomedical applications and, ultimately, organ replacement.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM4722223 and KGM5362212), a National Research Foundation (NRF) grant funded by the Korean government (MSIT) (NRF-2022R1A2B5B02001644), and a grant (22213MFDS386) from the Ministry of Food and Drug Safety, Korea, in 2022.
Data availability
Please contact the corresponding author for data availability.