Telencephalic organoids as model systems to study cortical development and diseases
Article information
Abstract
The telencephalon is the largest region of the brain and processes critical brain activity. Despite much progress, our understanding of the telencephalon’s function, development, and pathophysiological processes remains largely incomplete. Recently, 3-dimensional brain models, known as brain organoids, have attracted considerable attention in modern neurobiological research. Brain organoids have been proven to be valuable for studying the neurodevelopmental principles and pathophysiology of the brain, as well as for developing potential therapeutics. Brain organoids can change the paradigm of current research, replacing animal models. However, there are still limitations, and efforts are needed to improve brain organoid models. In this review, we provide an overview of the development and function of the telencephalon, as well as the techniques and scientific methods used to create fully developed telencephalon organoids. Additionally, we explore the limitations and challenges of current brain organoids and potential future advancements.
Introduction
The telencephalon comprises major areas in the mammalian brain with several important components, including the cerebral cortex, limbic system, basal ganglia, and the olfactory system [1,2]. The development of the telencephalon, which has several distinct parts, requires an interplay of diverse signaling pathways that are tightly regulated from the embryonic to the adult stages. In addition, various diseases related to telencephalon development arise due to genetic mutations or external factors [3]. Despite significant progress over the past decades in uncovering the mechanisms of brain development and pathophysiology, the intricate structure and function of the brain present a major challenge. Recently, models known as brain organoids have been developed to mimic the developing human brain [4]. Brain organoid technologies are excellent platforms for probing brain development, pathophysiology, and mechanisms. In this review paper, we will provide a brief overview of telencephalon development and telencephalon brain organoids, and we will also discuss the limitations of the current organoid system and future perspectives.
The telencephalon: an overview
Ethics statement: This study constituted a comprehensive analysis of previously released studies and thus, was not subject to the approval of the institutional review board.
The forebrain, also called the prosencephalon, comprises the largest part of the brain. It plays a key role in sensory processing, perception, and cognitive functions related to information processing [5,6]. The forebrain is divided into 2 regions: the telencephalon and the diencephalon. The telencephalon occupies the largest part of the central nervous system [7]. It is responsible for olfactory processing as well as speech, language, and memory formation. The main component of the telencephalon is the cerebral cortex, which is further divided into 4 lobes: the frontal, parietal, occipital, and temporal lobes [6]. The diencephalon is divided into 3 parts (the thalamus, epithalamus, and subthalamus) and has the function of maintaining homeostasis in the body [7]. The hypothalamus arises developmentally from the telencephalon, while anatomically it is adjacent to the regions from the diencephalon. Overall, the telencephalon and diencephalon have different roles, but they closely interact to perform their essential functions.
The frontal lobe, which is located at the front of the cerebrum, governs higher mental functions such as memory, thinking, and reasoning. It processes information from other association areas and regulates behavior. Damage to the frontal lobe often leads to a loss of problem-solving abilities and the capacity to plan and execute actions, such as crossing a street or answering complex questions [8,9]. Frontal lobe syndrome is a common condition associated with this area, and there are instances where a person’s behavior or personality changes due to trauma or various diseases [10]. The temporal lobe is the lateral part of the cortex. The right temporal lobe controls the left side of the body, and the left temporal lobe controls the right side. Functionally, it is mainly responsible for auditory stimulation, language, and emotional response. The medial temporal lobe includes the amygdala and hippocampus, which are the main structures forming the limbic system and play a critical role in memory function [11]. Temporal lobe epilepsy is one of the most common types of epilepsy in adults, and it is most frequently caused by sclerosis of the medial temporal lobe, particularly the hippocampus [12]. The occipital lobe is found at the back of the cerebral cortex and is the smallest lobe. Its functions primarily involve processing visual information coming from the eye. The primary visual cortex is the visual center of the occipital lobe. Visual information processed here is divided into 2 pathways: one towards the parietal lobe and the other towards the temporal lobe. The dorsal pathway to the parietal lobe processes visual information about moving objects, such as position, speed, and distance, as well as information about eye and body movements. The ventral pathway to the temporal lobe is responsible for judging the color and shape of the object being viewed by comparing it with existing images, and contributes to the long-term storage of visual memory [13]. Balint’s syndrome, characterized by severe spatial deficits and neuropsychological disorders, is a representative disease associated with this lobe [14]. The parietal lobe, which is located just behind the central sulcus in the cerebral cortex, is responsible for perceiving tactile and spatial senses and responding to the movement of objects in sight. It also integrates information from the outside world, combining letters into words to give them meaning [15,16]. Gerstmann syndrome, which causes learning disabilities and cognitive impairment, is a condition that can occur when the parietal lobe is damaged [17].
Early telencephalon development: a tale of signaling in neurogenesis.
The human brain development process consists of the generation, migration, and differentiation of neurons, as well as the maturation and formation of synapses [18]. Inhibitors of bone morphogenic protein (BMP), secreted from the organizer, induce the ectoderm to transform into nervous tissue, thereby forming a neural plate through morphogenesis [19]. The neural plate then folds in on itself to form the neural tube, with dorsal/ventral and anterior/posterior fates being patterned by the collective influence of signaling molecules (Fig. 1) [20].

Development of the neural tube. (A) Three primary vesicles in neural tubes develop into diverse brain areas. Expression of morphogens, fibroblast growth factor (FGF) 8, sonic hedgehog (Shh), and Wnt in neural tubes. (B) Morphogen gradients to specify the developmental axis for dorsal/ventral and rostral/caudal axis.
In the early stage of embryonic development, the central nervous system is subdivided into the forebrain, midbrain, hindbrain, and spinal cord along the anterior-posterior axis [21]. Various signaling pathways mediate this anterior-posterior patterning. The telencephalon originates from cells at the rostral part of the neural plate [22]. Wnts are known to play an important role in rostral-caudalization; thus, proper regulation of the interaction of Wnts and their antagonists is crucial in the establishment of the telencephalon [23]. Once the anterior and posterior of the neural plate are formed, the telencephalon undergoes dorsal-ventral patterning [24]. The telencephalon in mammals develops as the pallium of the dorsal region and the subpallium of the ventral region by dorsal-ventral patterning. The pallium is further divided into 4 regions: dorsal, medial, lateral, and ventral [25]. The dorsal pallium develops into the neocortex, which has the most complex structure in mammals. The medial pallium gives rise to the medial entorhinal cortex, hippocampus, cortical hem, and choroid plexus. The insular cortex and the lateral entorhinal cortex are known to originate from the lateral pallium. The ventral portion at the pallial-subpallial boundary gives rise to the amygdala [26–28]. In contrast, lateral, medial, and caudal ganglionic eminences (LGE, MGE, and CGE) in the subpallium develop as the basal ganglia [29]. Specific domains of the early telencephalon produce distinct sets of neurons and eventually generate the neural network of the mature telencephalon.
The regional patterning of the telencephalon is regulated by various morphogens, including Wnt, BMP, retinoic acid (RA), sonic hedgehog (Shh), and fibroblast growth factor (FGF). BMP or Wnt signaling is required for dorsal patterning of the telencephalon, while Shh signaling is important for ventral patterning, and RA is crucial for lateral patterning [22,25,30]. Wnts are expressed at the dorsal midline of the telencephalon, known as the cortical hem. Wnt signaling is important for patterning the medial pallium, which later develops into the hippocampus and medial entorhinal cortex [26,31]. When Wnt3a is knocked out and Wnt signaling is lost, the mouse hippocampus does not develop normally [32,33]. Furthermore, the hippocampus develops poorly in LEF1-knockout mice, a transcription factor known as a target gene for Wnt/β-catenin signaling [34]. BMPs are secreted from the lateral edges and dorsal midline of the neural plate. They enhance dorsomedial identity and the development of the choroid plexus [35,36]. The Shh signaling pathway is mediated by Smoothened, which specifies the ventral telencephalon [37]. The major source of Shh is the cells of the floor plate in the neural tube [38]. Under tight control of the gradient of Shh signaling, the ventral telencephalon gives rise to various ganglionic eminences (medial, caudal, and lateral) [39,40]. The ventral telencephalon in Shh-knockout mice displays the absence of expression of the telencephalon ventral markers Nkx2.1, Dlx2, and Gsx2 in neural progenitor cells [41].
Brain organoids: in vitro models of the human brain telencephalon
Over the years, researchers have actively used neural stem cells and neurons in cell culture systems, typically in 2-dimensional (2D) culture formats, or animal models to study the neural system. The use of cells in 2D culture is straightforward for addressing questions directly related to intrinsic cell features. However, this approach does not account for the interaction between the cells and the extracellular substrate found in tissue. Furthermore, the cerebral cortex is primarily composed of 6 laminar layers, a complexity that 2D culture cannot replicate [42]. Animal models have limitations in reproducing human responses due to species differences, necessitating new model systems.
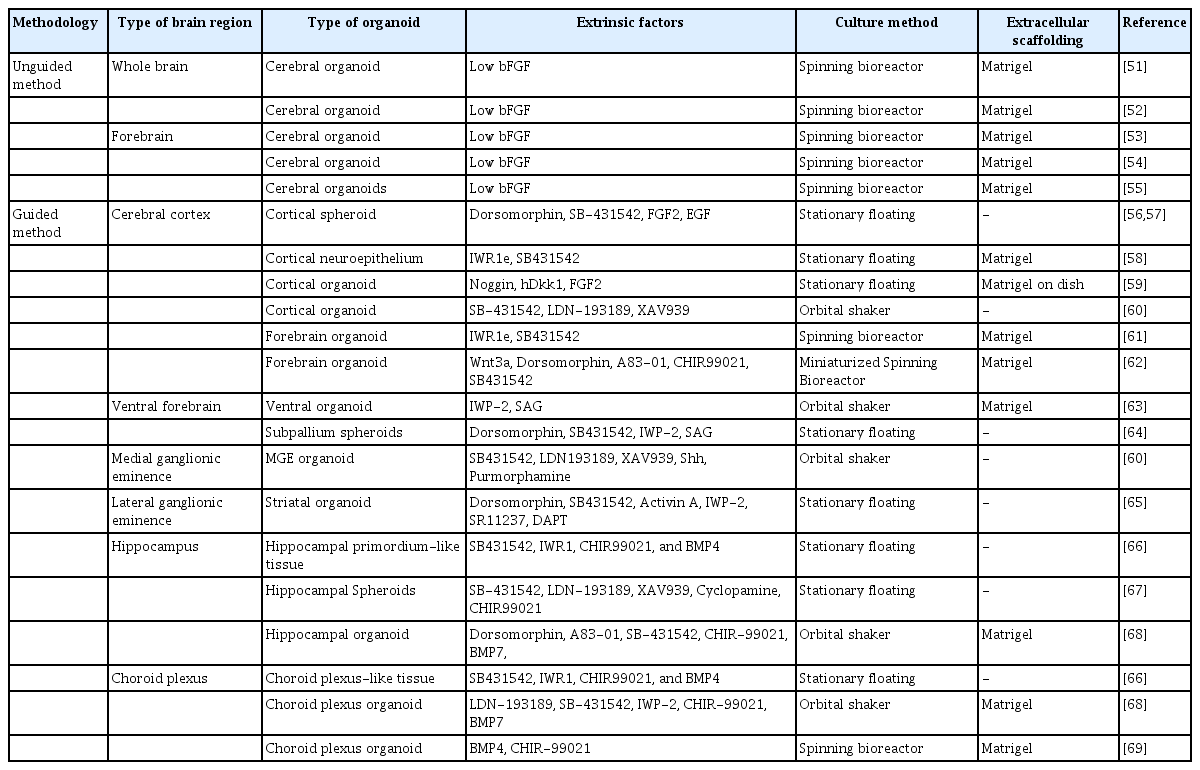
The establishment of stem cell culture conditions and 3-dimensional (3D) culture techniques enabled the development of brain organoids (Fig. 2) [43]. Organoids are constructed by culturing self-organizing cells with multicellular structures that represent complex in vivo cellular behavior and interactions. One of the main advantages of organoids is that they are much more similar to organs or tissues than conventional 2D cultured cells, while experimental approaches are much simpler than in animal models [44]. In general, brain organoids are differentiated by 2 approaches with distinct patterning steps: guided versus unguided. Unguided organoids are generated using minimal exogenous factors. This method was first attempted by the Knoblich group, who embedded 3D neuroepithelial spheroids in Matrigel and spinning environments to replicate the development of the human brain [45]. Another method is the guided method, which adds exogenous factors such as FGF, Wnt, BMP, RA, and Shh to produce brain organoids with a specific regional identity [46-49]. The guided approach was first tried by the Sasai group [50]. Table 1 summarizes the methods of generating brain organoids related to telencephalic regions [51–69].

Schematics to generate brain organoids. (A) General steps for generating brain organoids. Methods of culturing organoids in 3-dimensional (3D) and maintaining the organoids in 3D are shown. (B) The unguided approach to produce whole-brain organoids, or cerebral organoids, and the guided approach to produce regionally defined brain organoids. hPSC, human pluripotent stem cell; EB, embryoid body; NE, neural ectoderm.
Dual-SMAD inhibitors for the transforming growth factor-beta (TGF-β)/Activin/Nodal and BMP pathways have been widely used to induce neural differentiation [70,71]. The TGF-β/Activin/Nodal pathway is essential for self-renewal and endoderm differentiation, and the BMP pathway regulates mesodermal differentiation. Upon the inhibition of these pathways, pluripotent stem cells undergo neuroectoderm differentiation. The neural progenitors further acquire the regional identities according to the given regionalization cues [72]. During brain development, the regional identity of each brain region is established by patterning the neural tube with morphogens secreted from the organizers [73]. Strategies to generate region-specific brain organoids have been reported by applying these principles. To achieve telencephalic identity, an antagonist of Wnt signaling, a caudalizing factor, is used together with dual-SMAD inhibitors [46,72]. Several groups have used SMAD inhibitors and Wnt inhibitors to generate cortical organoids that resemble the dorsal telencephalon identity [74,75]. These cortical organoids are mainly composed of glutamate neurons, as well as progenitor cells and glial cells.
In addition, organoids representing specific telencephalic regions other than the cortex have been reported based on the combined use of patterning molecules such as BMP4, Wnt, and Shh. The cortical hem plays an important role in the development of dorsomedial telencephalic tissues such as the hippocampus, choroid plexus, and entorhinal cortex by providing Wnt and BMP [26,31,76]. In 2014, the Sasai group generated floating EB-like aggregates (SFEBq) resembling the hippocampus and choroid plexus using BMP4 and the Wnt activator CHIR99021. To induce the medial pallium fate, specific durations and doses of CHIR99021 and BMP4 were used, and organoids with cellular identities for the hippocampus and choroid plexus were generated. However, the choroid plexus-like structure in SFEBq was not shown to produce cerebrospinal fluid (CSF) [66]. A recent study on the generation of a choroid plexus organoid reported the generation of a CSF-like fluid similar to the in vivo choroid plexus [69]. More recently, the Ming group generated a choroid plexus expressing TTR, AQP1, and OTX2 by using high doses of BMP7 and CHR99021. These organoids were also used to model the impact of SARS-CoV-2 infection in disrupting the barrier integrity of the choroid plexus [68]. Hippocampus organoids were also generated by treatment with CHIR99021 alone [67].
The telencephalic dorsal-ventral axis identity is determined by temporal and spatial regulation of Shh signaling, which is a well-known ventralizing factor [77]. A few studies have successfully generated cortical organoids with ventral identity. Xiang et al. [60] used Shh and the Shh agonist purmophamine to generate ventralized telencephalic organoids resembling MGE. Specifically, this organoid showed a population of interneurons expressing somatostatin, which is produced specifically in the MGE [60,78]. Cederquist et al. [79] engineered an inducible Shh-expressing hPSC line to generate forebrain organoids. With proper modulation of Shh signaling, telencephalic organoids featuring dorsal and ventral regions were successfully generated. Miura et al. [65] reported organoids resembling the LGE by using activin A, IWP-2, and the retinoid X receptor (RXR) agonist SR11237. They also observed the projections between cortical and striatal neurons by generating morphologically and functionally mature LGE organoids. These organoids representing the ventral subpallium of the telencephalon can serve as an important tool to understand the properties of brain regions and to investigate networks between brain regions. However, organoids resembling the amygdala, entorhinal cortex, and CGE have not been reported yet.
Telencephalic organoids for models of brain diseases
Organoids were introduced decades ago, but they were replaced with cell culture systems [80,81]. However, the research and use of organoids have recently experienced a resurgence. They are now recognized as valuable tools in biomedical science, with applications ranging from basic research to therapeutic use. Organoids serve as excellent resources for studying organ development, maintaining homeostasis, and promoting regeneration. They are also useful in disease modeling, therapeutic development, and regenerative treatment through organoid transplantation [82]. Among the various types of organoids, brain organoids are unique in that they provide non-regenerating tissue. While the therapeutic potential of brain organoids is still being explored, they have already proven to be valuable resources for basic research on human brain development and diseases. Several brain disorders related to the telencephalon have recently been modeled (Table 2) [45,59,62-64,67,83–100].
Brain organoids can serve as a platform for the study of infectious diseases and host-pathogen interactions. For instance, a viral infection of the brain can be reproduced simply by infecting a brain organoid with the given virus. Virology techniques, immunofluorescence imaging, and single-cell RNA sequencing can be directly applied to the infected organoids to investigate the virus-host interaction. One of many examples includes modeling microcephaly by infecting the organoid with the Zika virus. Human brain organoids infected with the Zika virus exhibited growth inhibition, and a protein known as Zika-NS2A was found to inhibit the proliferation of radial glial cells [83]. Recently, cerebral organoids infected with the SARS-CoV-2 virus displayed hyperphosphorylation of tau and neuronal cell death. Interestingly, an abnormal tau distribution from the axon to the soma was observed [89]. These results provided intriguing insights into the developmental disorders and neurotoxicity caused by the virus.
Fragile X syndrome is a leading cause of both autism and intellectual disability. In forebrain organoids derived from fragile X syndrome iPSCs, there was an overexpression of CHD2, a gene associated with autism. Notably, treatment with a PI3K inhibitor was able to reverse these phenotypes [100]. Furthermore, a successful model of Timothy syndrome, a neurodevelopmental disorder characterized by autism spectrum disorder and epilepsy, was created using an assembloid system. This system combines 2 types of forebrain organoids, one representing the dorsal pallium and the other representing the subpallium. This model enabled the observation of interneuron migration from the subpallium to the pallium, revealing defects in the migration of intermediate neurons and an increase in residual calcium. Importantly, treatment with an L-type calcium channel blocker significantly reversed the neural and molecular phenotypes [64].
Alzheimer disease (AD) is a devastating neurodegenerative disorder that affects memory, thinking, and behavior. Although the exact pathogenesis and cause of AD have yet to be conclusively established, excessive accumulation of β-amyloid plaques or tau tangles are known culprits of AD [101]. Recently, AD models using human-induced pluripotent stem cells (hiPSCs) derived from patients with familial AD or Down syndrome have been developed [93]. Here, AD cerebral organoids showed an accumulation of β-amyloid peptides. Although the cortex is predominantly affected in AD, the hippocampus is the first region to show signs of the disease. To investigate the impact of AD pathogenesis on the hippocampus, hippocampus organoids from hiPSCs from AD patients carrying variations in the amyloid precursor protein or presenilin 1 (PS1) genes were reported. AD hippocampal organoids revealed that overexpression of NeuroD1 altered the expression of diverse genes, consequently affecting synaptic transmission [67]. Macrocephaly is a condition characterized by an abnormally large head size, and mutations in the tumor suppressor PTEN are a well-established genetic cause of this condition. In a macrocephaly model using brain organoids with PTEN knocked out, researchers observed increased organoid proliferation and surface area, effectively mimicking the characteristics of macrocephaly [97].
In addition to disease modeling, brain organoids generated from patient-derived hiPSCs enable customized drug screening. The patient’s data from next-generation sequencing, such as the whole genome, transcriptome, epigenome, as well as proteome and metabolomes, provide information for a deep understanding of the patient. Rett syndrome is an X chromosome-related neurodevelopmental disorder, and it is known that methyl CpG binding protein 2 (MeCP2) is the cause of genetic abnormalities. Our recent studies found that JQ1, a BET protein inhibitor, rescued abnormal neural activity and soma size, as well as the entire transcriptome in brain organoids with MeCP2 mutations [96]. There have also been reports of antitumor drug discovery using organoid-derived glioblastoma [102,103]. Numerous studies are currently underway to test the efficacy of drugs for specific diseases using organoid models.
Current limitations, challenges, and future perspectives
Brain organoids’ similarity to the actual brain has enabled disease modeling and the investigation of pathogenesis in brain disorders caused by genetics, infection, and cancer. These organoids have offered groundbreaking opportunities to study the developmental processes of the brain. However, there are still several limitations that need to be addressed.
One of the main challenges is controlling the quality and homogeneity of organoids. Even when the same stem cell line is utilized, each organoid may not exhibit identical structure and developmental timing [43]. This issue directly impacts the reliability of organoid model systems in pharmaceutical drug development and diagnostics. To overcome this limitation, attempts have been made to improve organoid reproducibility and homogeneity by applying bioengineering tools. For example, synthetic microfluidic systems produce more homogeneous and reproducible organoids through the precise control of experimental parameters [104]. In addition, single-use vertical wheel bioreactors generated reproducible, scalable, and homogeneous mature organoids [105]. Micropillar array technology is another method for controlling organoid size and increasing reproducibility [106]. These engineering techniques may become the gold standard for consistently producing homogeneous brain organoids.
Another major challenge in brain organoids is achieving neural maturity. Although many studies characterize brain organoids by documenting neural activity, only a select few neurons exhibit significant activity. The root cause of this neuronal immaturity is often attributed to an insufficient development period for in vitro brain organoids. In fact, recent studies that conducted longitudinal neural activity measurements for long-term culture organoids have demonstrated that early organoids display less neural activity, which gradually matures with further development. Moreover, brain activity in organoids over a year old exhibited irregular electroencephalogram patterns, similar to the chaotic bursts of synchronized electrical activity observed in the developing brains of premature infants. This rhythm was comparable to that of infants born 25 to 39 weeks post-fertilization [107]. In summary, while it is now possible to replicate the immature brain in brain organoids, further research is required to accurately reproduce the mature adult brain.
Tissue comprises complex microenvironments that coexist with a variety of cell types. However, brain organoids do not possess such intricate structures and often lack these microenvironments. Various non-neuronal cells also play a crucial role in the development and function of the nervous system. For instance, endothelial cells, pericytes, and microglia are non-neuronal cells that are integral to vascular systems [108,109]. The lack of these systems can lead to cell apoptosis in brain organoids due to the failure to supply nutrients or oxygen to the organoid's inner core 54. Furthermore, brain organoids lack essential resident immune cells known as microglia. Recently, methods have been developed to address these limitations [110,111]. Cakir et al. [112] generated cortical organoids with vascular-like structures using embryonic stem cells that ectopically expressed the human E26 transformation specific (ETS) variant transcription factor 2 (ETV2). These cortical organoids with vascular-like structures demonstrated blood-brain barrier properties, including an increase in the expression of tight junctions and nutrient transport. In another approach, Shi et al. [113] developed a protocol to generate vascularized cortical organoids by co-culturing human embryonic stem cells with human umbilical vein endothelial cells (HUVECs). These HUVECs formed a well-developed vascular system in the brain organoids, enabling long-term culture for over 200 days. In addition, Ormel et al. [114] succeeded in generating cerebral organoids containing microglia. Microglia are known to play a significant role in the brain's immune system and neuronal maturation. Organoids containing microglia could greatly aid disease research and provide opportunities to explore the in-depth role of microglia in brain development. However, systems with various non-neuronal cells are still not fully integrated into brain organoids, and the verification of their precise functions remains incomplete. The absence of such systems may also pose limitations in the disease modeling of brain organoids.
Reconstructing the interaction between different brain regions remains a significant challenge in the field of brain organoids. Most brain organoids developed to date represent only specific regions. To address these challenges, the assembloid system has been introduced. This system combines multiple region-specific organoid types to recreate the interaction between the given regions. Examples of this include the assembly of dorsal cortical organoids with ventral cortical organoids, and thalamic organoids with cortical organoids [60,74]. The assembloid method can be used to directly establish a disease model and illustrate the abnormal regulation of neural circuits in neuropsychiatric disorders, such as Timothy syndrome [65]. This approach facilitates the study of biological mechanisms that require interactions between various brain organoid regions in vitro, and also serves as a platform for disease modeling.
Organoids are among the most accessible and physiologically suitable models to study the differentiation process of stem cells in controlled environments. Brain organoids have been proven to be a high-fidelity platform to reveal the unique identity of stem cells and the niche composition of the surrounding microenvironment [115]. Combined with analyses of genetic information, the transcriptome, and proteins, organoids have significantly contributed to our understanding of brain development, homeostasis maintenance, and key aspects of disease. The process of creating a brain organoid that mirrors human brain development has been utilized in various ways, ranging from basic research tools to applied research. Brain organoids facilitate disease modeling and pathogenesis studies for conditions such as infectious diseases, genetic diseases, and cancer, thereby aiding in the identification of reliable molecular targets [81,116]. When combined with an engineering approach, brain organoids can serve as testing grounds for evaluating drug efficacy and toxicity. The integration of organoid technology with current technologies leads to a variety of subsequent functions and applications, underscoring the versatility of organoids (Fig. 3). These features, coupled with their physiological relevance, position organoids as one of the most exciting and promising technologies recently introduced for studying human brain development, disease, and treatment.

Application and utility of 3-dimensional brain organoids. (A) For basic research, fused organoids or assembloids are used to study neural connectivity. Integrating with non-neuroectodermal cells to investigate microglia and endothelial cells. (B) For disease modeling, brain organoids can be used to study neurodevelopmental and neurodegenerative disorders. hPSC, human pluripotent stem cell; NE, neural ectoderm.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
We thank Hyunjin Lee for the illustration. In-Hyun Park was partly supported by NIH (R01MH118344-01A1). W.Y was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education(2021R1A6A3A14043824).
Authors’ contributions
Conceptualization: WSY, IHP; Project administration: IHP; Visualization: WSY; Writing–original draft: WSY, FRK; Writing–review & editing: WSY, IHP.
Data availability
Please reach out to the corresponding author to inquire about the availability of data.