|
 |
| Organoid > Volume 4; 2024 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Supplementary Information
Fig. S1.
NOTES
Conflict of interest
Hyun-Jeong Ko has been the Editorial Board member of the Organoid Society since 2024. No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Education and ICT and Ministry of Science) (RS-2023-00208385) and Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2022R1A6C101A739).
Fig. 1.
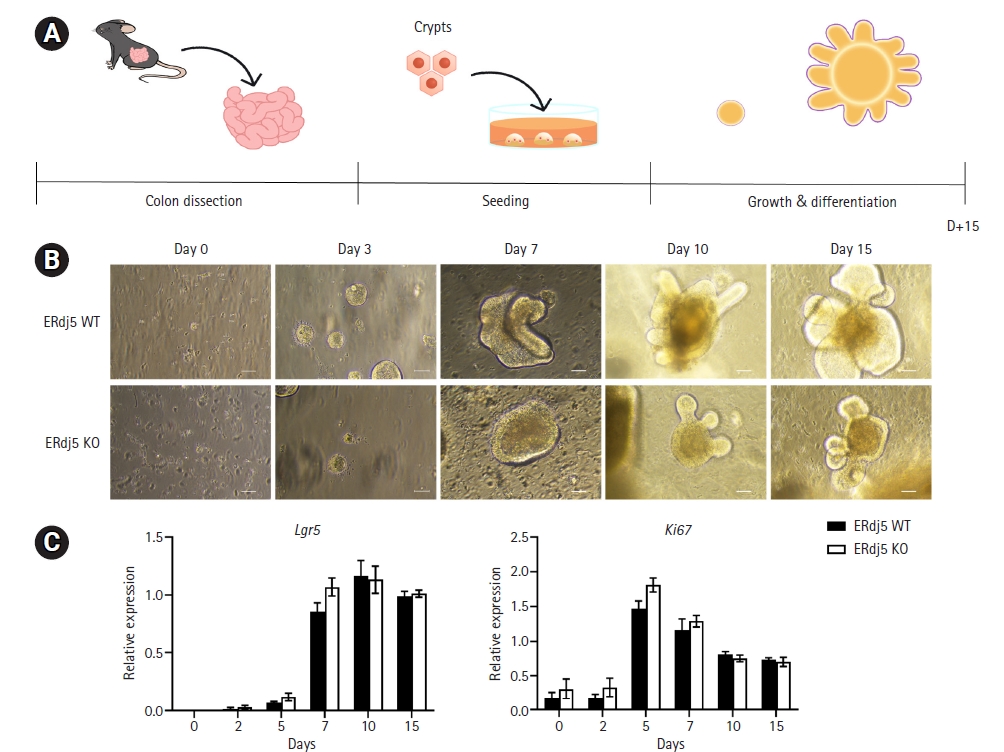
Fig. 2.

Fig. 3.

Fig. 4.
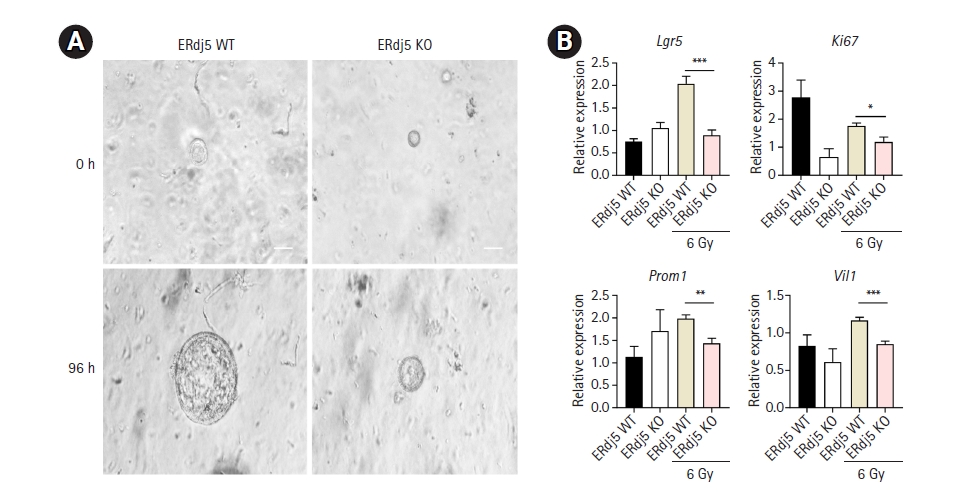
Table 1.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 614 View
- 9 Download
- ORCID iDs
-
Hyunjin Jeong

https://orcid.org/0000-0003-4386-3845Jaewon Cho

https://orcid.org/0000-0003-0766-8858Hyun-Jeong Ko

https://orcid.org/0000-0002-3844-928X - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print