Small molecule-induced destabilization of β-catenin and RAS is the ideal strategies for suppressing colorectal cancer
Article information
Abstract
Background
Mutations of adenomatous polyposis coli (APC) and KRAS play essential roles in the development of colorectal cancer (CRC) by forming an abnormal colon morphology. Despite intensive efforts to discover therapeutic strategies to re-transform cancer cells into normal cells, no effective approaches have been reported yet.
Methods
In this study, we aimed to identify therapeutic strategies for inducing morphological changes of tumor organoids to structures similar to the normal intestine in ApcMin/+/KrasG12DLA2 mice by using KYA1797K, a dual inhibitor of the Wnt/β-catenin and RAS signaling pathways.
Results
KYA1797K, previously identified as a dual inhibitor of the Wnt/β-catenin and RAS pathways, inhibited the growth of organoids derived from tumor cells of ApcMin/+/KrasG12DLA2 mice, with the transformation of benign tumor structures into normal structures, similar to bone morphogenetic protein 4 (BMP4), an intestinal differentiation signaling inducer.
Conclusion
Given the anti-cancer effects of KYA1797K and its ability to induce morphological changes similar to those elicited by BMP4 treatment, the dual suppression of Wnt/β-catenin and RAS signaling is a potential therapy for treating CRC.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the entire world [1], and it has been the second leading cause of cancer-related deaths as a consequence of recurrence and metastasis [2]. Due to adenomatous polyposis coli (APC) (90%) and KRAS mutations (50%), the Wnt/β-catenin and Ras-ERK pathways are abnormally activated and promote tumor progression, contributing to recurrence and metastasis in advanced CRC [3–6], by regulating intestinal homeostasis through the maintenance of intestinal stem cells [7,8] and differentiation [7,9–12]. APC and KRAS mutations synergistically result in tumor development and the activation of cancer stem cells (CSCs) [3,5], which constitute a sub-population within tumor cells presenting the properties of stem cells [13–15]. Consequently, simultaneous suppression of both the Wnt/β-catenin and RAS pathways has been considered as a potentially effective therapeutic strategy to inactivate CSCs for treating advanced CRC [16–18].
Bone morphogenetic protein (BMP) signaling, comprising BMP ligands and transforming growth factor-β family members, plays essential roles in the differentiation and proliferation of CSCs in CRC by balancing the Wnt signaling mediated-homeostatic self-renewal [19]. The secretion of BMP ligands induces the differentiation and maturation of epithelial cells through paracrine signaling pathways [6,8,20]. Specifically, as a key negative regulator of Wnt signaling, bone morphogenetic protein 4 (BMP4) is expressed by mesenchymal cells along the colon-crypt axis from top to bottom. Furthermore, the distribution of BMP4 by its signaling pathways increases toward the top of intestinal epithelium in the crypt [19,21]. Conversely, suppressing BMP4 activity leads to the altered maturation of epithelial cells and CRC development [22]. The most convincing evidence for the role of BMP4 in CRC implies that the inactivation of the BMP pathway in genetically modified mouse models may be a major factor in tumorigenesis and polyp formation of CRC, with upregulation of the Wnt signaling pathway [23–25]. BMP4 treatment has been shown to stimulate the differentiation, apoptosis, and maturation of CSCs by reducing Wnt/β-catenin signaling [26–28]. Accordingly, the suppression of Wnt/β-catenin signaling has a potent therapeutic effect against CRC stem cells via the activation of BMP signaling pathways, leading to the differentiation of normal-appearing structures.
Previously, therapeutic effects of KYA1797K treatment on CSC inhibition have been evaluated in genetic animal models of CRC, patient-derived tumor organoids, and xenografts [29]. We observed that the inhibition of Wnt/β-catenin and RAS pathways by KYA1797K treatment effectively suppressed CRC tumorigenesis, with decreases in CSC activity and population as determined by the level of LGR5 as a CSC marker and precursor markers such as CD44, CD133, and CD166 [18]. However, the morphological changes characteristic of the differentiation of CRC stem cells have not been investigated. In this study, we assessed morphological changes in organoids derived from tumor cells of ApcMin/+/KrasG12DLA2. Similar to BMP4 treatment, which is known to induce differentiation in CRC stem cells, KYA1797K treatment effectively promotes the transformation of tumor cells into normal-appearing structures with potent anti-cancer effects. Taken together, we suggest that the simultaneous destabilization of both β-catenin and RAS by KYA1797K provides a potential therapeutic approach to treat advanced CRC and improve clinical outcomes.
Materials and Methods
Ethics statement: This study was not performed by Human resources. Therefore, exempt from institutional review board approval.
1. Reagents and drug treatment
KYA1797K was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA). Unless otherwise indicated, KYA1797K was used at a concentration of 25 μM. Recombinant BMP4 (rBMP4) was obtained from R&D Systems (Minneapolis, MN, USA). rBMP4 was used at 20 ng/mL unless otherwise stated.
2. Animal studies and tumor organoid experiments
All steps of the animal experiments were performed in accordance with the guidelines of the Korean Food and Drug Administration. All steps and protocols were approved by the Institutional Animal Care and Use Committee of Yonsei University. C57BL6J-ApcMin/+ (ApcMin/+) and B6.129S-Krastm3Tyj (KrasG12DLA2) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). To obtain ApcMin/+/KrasG12DLA2 mice for the tumor organoid experiments, we crossed ApcMin/+ mice with KrasG12DLA2 mice. The mouse genotype was confirmed by the genomic DNA isolation from the tail. To control for genetic background effects, sex-matched littermates were utilized as controls.
For the experiments involving tumor organoids, small intestinal tumors were isolated from ApcMin/+/KrasG12DLA2 mice. The tumors washed with ice-cold phosphate-buffered saline, and the isolated single cells from tumors were collected using trypsin solution (0.25%) (Gibco, Carlsbad, CA, USA) with 10 μM Ly27632 (Sigma-Aldrich) and 100 μg/mL Primocin (Invivogen, San Diego, CA, USA) for 30 minutes. After incubation with trypsin, B27 supplement (1×) (Sigma-Aldrich) was added, and the mixed solution was filtered through cell strainers (100 μm and 40 μm) (BD Biosciences, Bedford, MA, USA) to obtain single cells. Growth factor-reduced Matrigel (BD Biosciences) was gently mixed with the collected cells. The growth medium was prepared with N2 medium containing 50 ng/mL epidermal growth factor (Peprotech, Rocky Hill, NJ, USA), 100 ng/mL noggin (Peprotech), 1 μg/mL R-spondin-1 (Peprotech), 1.25 mM N-acetyl cysteine (Sigma-Aldrich), and 10 mM nicotinamide (Sigma-Aldrich). The growth medium was replaced with fresh medium every 2 days, and the cells were passaged by mechanical disruption every 10 to 14 days (1:5 ratios). For the treatment of rBMP4, after 3 days of culture, the medium was replaced with fresh medium containing 20 μg/mL rBMP4 (R&D systems). The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) was performed to measure tumor organoid growth with a FLUOstar Optima instrument (BMG Labtech, Ortenberg, Germany).
3. Statistical analysis
Values of all data are represented as the mean ± standard deviation of 3 independent experiments using GraphPad Prism 5 Software, as described previously [30]. The statistical significance of differences was analyzed using the Student t-test. Significance was denoted as not significant, p<0.05, p<0.01, and p<0.001.
Results
1. Poor survivals in CRC patients expressing low BMP4, high KRAS, and high CTNNB1
Advanced CRC is characterized by high expression of β-catenin and RAS resulting from genetic mutations [7,10,12]. Through the interconnection between Wnt/β-catenin and RAS in CRC, tumor development is consequently upregulated by the induction of differentiation, involving the loss of BMP4 activity [31]. To determine the clinical significance of BMP4, KRAS, and CTNNB1 (encoding β-catenin), we evaluated their associations with the prognosis of CRC patients using a public gene expression data set (GSE17536, n=177). As shown in Fig. 1A, low expression of BMP4 was associated with short overall survival (OS) in CRC patients. A high expression level of KRAS was significantly associated with lower OS, disease-free survival (DFS), and disease-specific survival (DSS) in comparison with patients who had low KRAS expression (Fig. 1B). A high expression level of CTNNB1 was also associated with lower OS, DFS, and DSS than in patients with low expression (Fig. 1C). Collectively, these results suggest that the BMP4, KRAS, and CTNNB1 genes have clinical importance for the survival rate with statistical significance (p<0.05). Furthermore, our strategy to destabilize β-catenin and RAS by modulating Wnt/β-catenin and BMP4 signaling could be a potent approach to improving the survival rate of CRC patients.

Evaluation of the prognosis and survival rate in colorectal cancer (CRC) patients based on gene expression in the GSE data set (GSE17536). The relationship of the gene expression of (A) BMP4, (B) KRAS, and (C) CTNNB1 with overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS) in CRC patients.
2. BMP4 induces the transformation of tumor cells into normal-appearing structures in CRC tumor organoids derived from ApcMin/+/KrasG12DLA2 mice.
To confirm the involvement of BMP4 in the morphological changes demonstrating the differentiation of CRC stem cells, we investigated whether BMP4 induced the transformation of tumor cells into normal-appearing structures in small intestinal tumor cells derived from ApcMin/+/KrasG12DLA2 mice. During 5 days of culture, the tumor organoids expanded with the unique structures of 3D cultured intestinal tumor organoids, which consist of a lumen surrounded by a lining of epithelial tumor cells. After 6 and 7 days of culture with rBMP4, we identified that the tumor organoids showed a significant tendency to form normal-appearing structures (Fig. 2A). On day 9, the morphological changes of tumor organoids to normal-appearing structures were promoted by treatment with rBMP4 (Fig. 2B). These results suggest that BMP4, as a differentiation inducer of CRC stem cells, significantly promoted the transformation of tumor organoids into normal-appearing structures in CRC.

Bone morphogenetic protein 4 (BMP4) induces the transformation of tumor cells into normal-appearing structures in colorectal cancer tumor organoids derived from ApcMin/+/KrasG12DLA2 mice. The tumor organoids derived from tumor cells in ApcMin/+/KrasG12DLA2 were cultured with N2 medium containing 50 ng/mL epidermal growth factor, 100 ng/mL noggin, 1 μg/mL R-spondin-1, 1.25 mM N-acetyl cysteine, and 10 mM nicotinamide. After 3 days, the medium was replaced with fresh medium containing 20 μg/mL recombinant BMP4 (rBMP4). (A) Effect of rBMP4 on tumor organoids derived from small intestinal tumor cells for each indicated day. At 5 days of culture, the tumor organoids showed the lumen surrounded by the lining epithelial tumor cell (red arrows) by treatment of rBMP4. At 6 days of culture, the tumor organoids showed a budding structure (white arrow) in response to treatment with rBMP4. At 7 days of culture, the tumor organoids showed normal-appearing structures (black arrows). after treatment with rBMP4. Scale bar=50 μm. (B) Comparison of the effects on transformation into normal-appearing structures (green arrow) between the control group (DMSO) and rBMP4-treated group on day 9. Using bright-field microscopy, representative images were captured using a Nikon 2000 U microscope from 3 different fields. Scale bar=50 μm.
3. Comparison of the effect on transformation of tumor cells into normal-appearing structures between KYA1797K and BMP4 in tumor organoids derived from ApcMin/+/KrasG12DLA2 mice
We investigated whether KY1797K showed an effect on the transformation of tumor structure into normal-appearing structure similar to that of BMP4 in the tumor organoids derived from ApcMin/+/KrasG12DLA2 mice (Fig. 3A). With 11 days of treatment, KY1797K significantly decreased the size of the tumor organoids, but had no significant effects on inducing the transformation of tumor cells into normal-appearing structures, unlike the results observed after treatment with rBMP4 (Fig. 3B). KY1797K significantly suppressed the number of tumor organoids on day 14 in a dose-dependent manner, unlike the presence of rBMP4 (Fig. 3C).
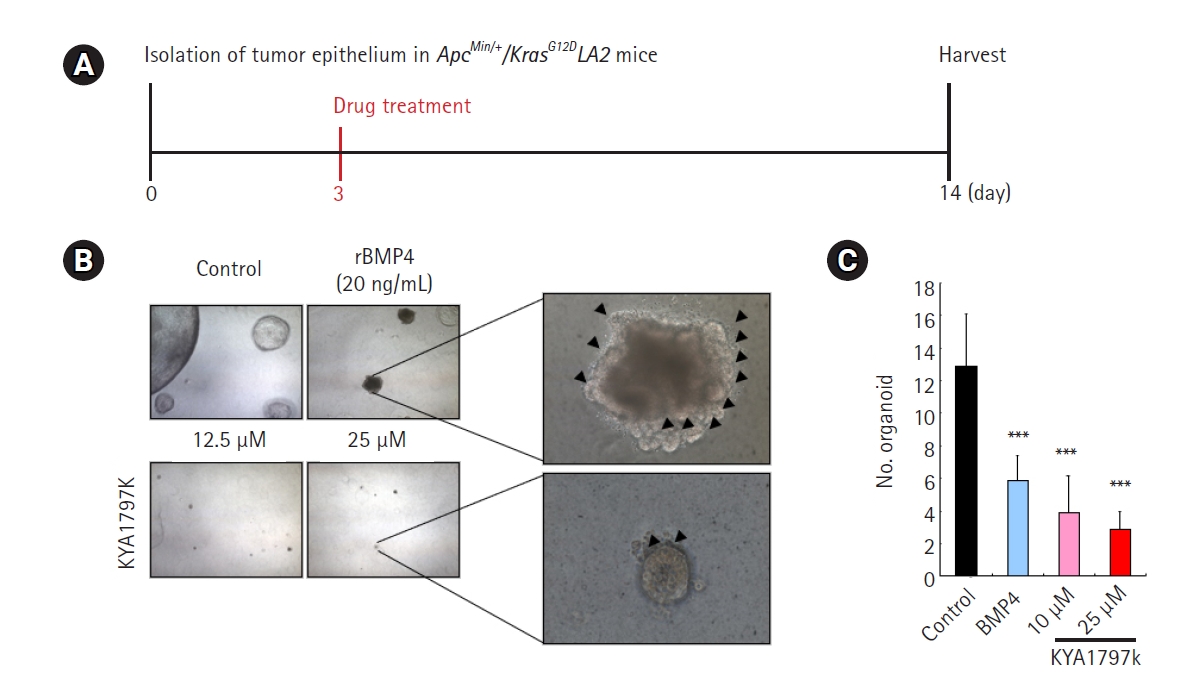
Comparison of the effect on the transformation of tumor cells into normal-appearing structures between KYA1797K and recombinant bone morphogenetic protein 4 (rBMP4) in tumor organoids derived from ApcMin/+/KrasG12DLA2 mice. Tumor organoids derived from tumor cells in ApcMin/+/KrasG12DLA2 mice were cultured with N2 medium containing 50 ng/mL epidermal growth factor, 100 ng/mL noggin, 1 μg/mL R-spondin-1, 1.25 mM N-acetyl cysteine, 10 mM nicotinamide. After 3 days, the medium was replaced with fresh medium containing 20 μg/mL rBMP4. (A) Timeline of culturing the tumor organoids with KYA1797K (11 days). (B) Comparison of the effects on the transformation into normal-appearing structures between KYA1797K and rBMP4 in CRC tumor organoids derived from ApcMin/+/KrasG12DLA2 mice (black arrowheads). Scale bar=50 μm. (C) The numbers of tumor organoids were measured using ImageJ software. Quantitative data are presented as mean±standard deviation. ***p<0.001.
4. Morpholigical changes into normal-appearing structures in KYA1797K-treated-tumor organoids derived from ApcMin/+/KrasG12DLA2 mice
To optimize the treatment conditions of KYA1797K, we investigated the effect of short treatment for 7 days with KY1797K in the tumor organoids derived from ApcMin/+/KrasG12DLA2 mice (Fig. 4A). As shown in Fig. 4B, a short treatment of KY1797K induced a significant transformation of tumor cells into normal-appearing structures, showing a similar effect to rBMP4, as presented in Fig. 2B and Fig. 3B. Moreover, KY1797K treatment significantly reduced the number of organoids (Fig. 4C) and promoted the anti-proliferative effect of tumor organoids (Fig. 4D). Taken together, these results suggest that the KYA1797K-induced dual suppression of Wnt/β-catenin and RAS inhibition promotes the transformation of tumor cells into normal-appearing structures, with potent anti-cancer effects.
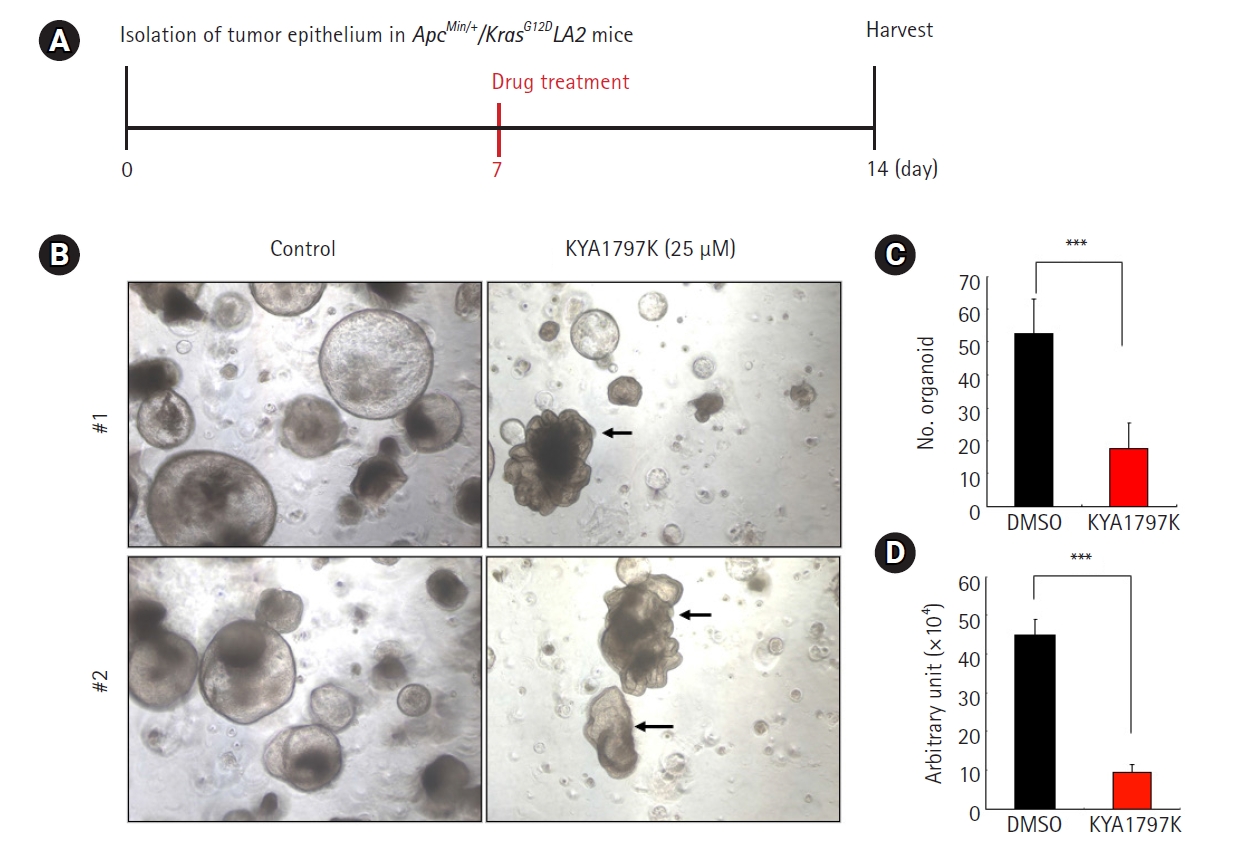
KYA1797K inhibited tumor growth and induced the transformation of tumor cells to normal-appearing structures in tumor organoids derived from ApcMin/+/KrasG12DLA2 mice. (A) The timeline of culturing the tumor organoids using the optimized condition of short treatment with KYA1797K (7 days). (B) The effects of KYA1797K in colorectal cancer organoids derived from ApcMin/+/KrasG12DLA2 mice, showing the transformation of normal-appearing structures (black arrow). Scale bar=50 μm. (C) The area and number of tumor organoids were measured using ImageJ software. The quantitative data are presented as mean±standard deviation. ***p<0.001. (D) Viable cells were measured by the Luminescent Cell Viability Assay. The quantitative data are presented as mean±standard deviation. ***p<0.001.
Discussion
In multiple cancer types, including CRC, the Wnt/β-catenin and RAS pathways show abnormal activation with upregulated expression of β-catenin and RAS proteins [30,32,33]. In CRC, due to the critical roles of the β-catenin and RAS proteins associated with the Wnt/β-catenin and RAS pathways, the frequently mutated genes activate each pathway and also affect cross-talk between the 2 pathways [34–36]. Both APC (90%) and KRAS (50%) mutations synergistically activate the Wnt/β-catenin pathway by stimulating the RAS/ERK pathways, involving the positive regulation of tumorigenesis in CRC [37,38]. Therefore, clear evidence from multiple studies has suggested that the small molecule-induced destabilizing expression of the β-catenin and RAS proteins may provide therapeutic opportunities for treating human CRC [39-40].
In this study, we observed that the KYA1797K-induced destabilization of both β-catenin and RAS effectively induced the transformation of tumor cells into normal-appearing structures with potent anti-cancer effects in small intestinal tumor organoids derived from ApcMin/+/KrasG12DLA2 mice in the presence of APC and KRAS mutations, including hyperactivation of the Wnt/β-catenin and RAS signaling pathways. Compared with the effects of rBMP4, the optimized condition of short treatment with KYA1797K demonstrated significantly similar effects, inducing the transformation of tumor cells into normal-appearing structures while showing simultaneous growth inhibition in CRC tumor organoids.
In conclusion, we demonstrate that the small molecule-induced destabilization of β-catenin and RAS via suppression of both the Wnt/β-catenin and RAS pathways could serve as a potential approach for treating CRC patients by changing the morphology of tumor organoids into normal-appearing structures and decreasing tumor growth. Considering the importance of morphological changes demonstrating the differentiation of CRC stem cells, this small-molecule approach would be a promising strategy for treating CRC patients with a subsequent improvement in clinical outcomes.
Notes
Conflict of interest
Yong-Hee Cho is working as a deputy editor from 2022 to 2023. However, Yong-Hee Cho was not involved in the review process of this manuscript.
Funding
This study was supported by a grant from the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science (grants:2021R1C1C2012601, 2022R1C1C2006078).
Author contributions
Conceptualization: YK, YHC; Data curation: YK, GWK, YHC, MHK; Funding acquisition: YK, YHC; Methodology: YK, YHC; Project administration: YHC; Investigation: YK; Resources: YHC, GWK; Software: YK, YHC, MHK; Supervision: YHC; Validation: YK, YHC; Writing-original draft: YK, YHC, MHK; Writing-review & editing: YK, YHC.
Data availability
Please contact the corresponding author for data availability.
