Standard operating protocol of hepatic organoid differentiation from human induced pluripotent stem cells
Article information
Abstract
Background
Mature liver organoids are potential cellular sources for research to understand the pathology of several liver-related conditions, including end-stage chronic liver disease. Although several methods exist for the differentiation of mature hepatic organoids from human induced pluripotent stem cells (hiPSCs), organoid generation have limitations due to various experimental culture conditions.
Methods
We differentiated homozygous hiPSCs into definitive endoderm, hepatic endoderm, and hepatic maturation in a two-dimensional culture condition. Expandable hepatic organoids (EHO) and mature hepatic organoids (MHO) were cultured in a three-dimensional culture condition.
Results
We successfully established hepatic organoids that were reproductively and expandably cultured for at least three months. MHO showed significant increases in hepatic-specific markers, drug-metabolizing enzymes and transporter genes, and human albumin secretion.
Conclusion
Therefore, we established a standard operating protocol for gen¬erating mature and expandable hepatic organoids derived from hiPSCs, and made the start¬ing materials available to promote widespread use of the protocol.
Introduction
Despite the liver’s regenerative capacity, organ transplantation is the only treatment available for chronic liver diseases, such as viral infections, resulting in end-stage liver disease [1]. However, the shortage of donors remains a serious limitation of transplantation, requiring further efforts to develop effective cell therapies. Liver organoids have been derived from primary liver tissues, which are expandable and functional [2,3].
Human induced pluripotent stem cells (hiPSC) have been highlighted as an alternative source for differentiation into various cell types, including hepatocytes [4,5], pancreatic islets [6,7], epicardial cells [8], and macrophages [9,10]. Since pluripotent stem cells (PSCs) were first isolated, efficient hepatic progenitor cells have been generated from PSCs derived from mice and humans [11,12]. For successful differentiation of human PSCs (hPSCs) into liver mimetics, 3-dimensional (3D) culture systems provide the ability to generate functional liver organoids [13,14] with maintained differentiation potential and metabolic function. Although Takebe et al. [15–17] reported functional liver buds generated from hiPSCs, the differentiated liver buds still manifested immature hepatic organoids and were not proliferative.
We recently reported the generation of expandable and mature hepatic organoids through a modified protocol based on liver development periods using a 3D system [18]. The hepatic organoids derived from hPSCs were proliferative and could be scaled up for mass production. Massive hepatic organoids offer a potential useful tool in research on disease modeling and drug stability. Despite the broad potential utility of hPSC-derived liver organoids, some labs have difficulties generating mature hepatic organoids and maintaining long-term cultures [13]. In this study, we aimed to establish a standard operating protocol of a reproducible liver organoid differentiation method based on our previous report [18]. This protocol resulted in the generation of mature hepatic organoids that can be applied in suitable hepatic models for drug toxicity, drug screening, and disease modeling. Furthermore, we are making the starting materials (KSCBi005) [19] available for broad distribution; the differentiation capability of this material was confirmed via the Korea National Stem Cell Bank (https://nih.go.kr/contents.es?mid=a50401110200).
Standard operating protocol of hepatic organoid differentiation from human iPSCs
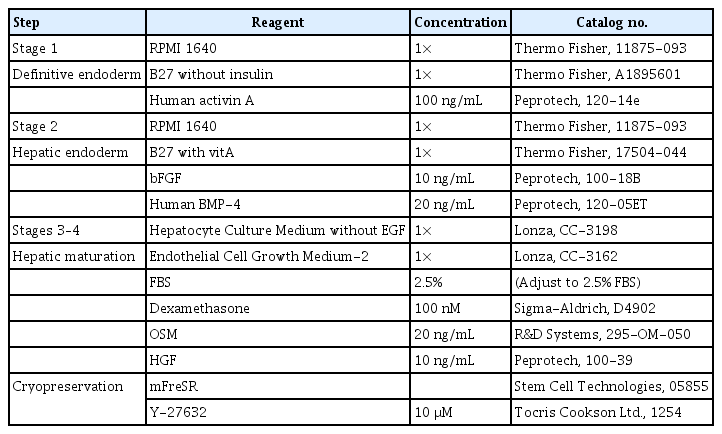
1. Stage 0: cell culture of hiPSCs
The hiPSC lines CMC-hiPSC-003 and CMC-hiPSC-009 (National Center for Stem Cell and Regenerative Medicine, Cheongju, Korea) were cultured on vitronectin (Gibco; Thermo Fisher, Waltham, MA, USA)-coated dishes and in mTeSR 1 medium (Stem Cell Technologies, Vancouver, BC, Canada). CRL-2097-hiPSCs were reprogrammed from human fibroblasts that were purchased from ATCC (CRL-2097). The cells were dissociated in Dulbecco’s phosphate-buffered saline (DPBS) with 0.5 mM EDTA (Gibco) for 5 minutes at 37°C. For the first 24 hours after splitting, 10 μM Y-27632 (Tocris Cookson Ltd., Bristol, UK) was added.
2. Stages 1–4: generation of hepatocyte-like organoids
hiPSCs were differentiated into hepatic buds in a 2-dimensional (2D) culture system, and the collected hepatic buds were differentiated into hepatic organoids and then mature hepatic organoids in Matrigel (Corning Inc., Corning, NY, USA) with a 3D culture system. The overall scheme of differentiation stages and the cell morphologies corresponding to each stage are shown in Fig. 1.

Scheme of differentiation for hepatic organoids (HOs) derived from human induced pluripotent stem cells (hiPSCs). (A) Overview of the differentiation protocol for HO generation. Scale bar=500 μm. (B) Representative morphology of organoids at each step of differentiation. EHO, expandable hepatic organoid; MHO, mature hepatic organoid; 2D, 2-dimensional; 3D, 3-dimensional. Scale bar=1 mm.
1) Stage 1: definitive endoderm
For cell differentiation, hiPSCs were seeded on Matrigel-coated plates and cultured in mTeSR 1 medium for 3 days until reaching 80% to 90% confluence. The hiPSCs remained in colonies. For definitive endoderm differentiation, the medium was changed to RPMI-1640 (11875-093; Thermo Fisher) supplemented with B27 minus insulin (A1895601; Thermo Fisher) and 100 ng/mL human activin A (120-14e; Peprotech, Cambridge, UK) for 6 days.
2) Stage 2: hepatic endoderm
Definitive endoderm cells were cultured in RPMI-1640 with B27 (17504-044; Thermo Fisher), 10 ng/mL basic fibroblast growth factor, and 20 ng/mL human bone morphogenic protein-4 (120-05ET; Peprotech) and incubated in 5% hypoxic conditions for 4 days.
3) Stage 3: mature hepatocytes
To induce differentiation to mature hepatocytes in a 2D culture system, the medium was replaced with Hepatocyte Culture Medium (CC-3198; Lonza, Basel, Swiss) without epidermal growth factor, mixed with Endothelial Cell Growth Medium-2 (CC-3162; Lonza, Basel, Swiss) (1:1), adjusted to a 2.5% fetal bovine serum concentration with 100 nM dexamethasone (D4902; Sigma-Aldrich, St. Louis, MO, USA), 20 ng/mL OSM (295-OM-050; R&D Systems, Minneapolis, MN, USA), and 10 ng/mL human hepatocyte growth factor (100-39; Peprotech) for 4 days and incubated in 5% hypoxia. The cells were then further cultured in normoxia for 7 days.
4) Stage 4: hepatic buds
The hepatic buds were generated 4 to 5 days after hepatic maturation in the 2D culture system, and were collected for further processing and analysis.
3. Stages 5–7: differentiation of mature hepatic organoids
1) Stage 5: hepatic organoids
Collected hepatic buds were solidified with Matrigel as a dome for 20 minutes in a 37°C incubator. Hepatic organoid medium was then gently added. Organoids were generally cultured in hepatic organoid medium, and the medium was replaced every 3 days. These organoids were passaged every 5 days, and were washed with cold DPBS and collected. The organoids were broadly spread on a new plate and mechanically cut into a grid pattern using a blade. The passaged organoids were plated with Matrigel at a 1:10 ratio, and then were preserved with mFreSR (05855; Stem Cell Technologies).
2) Stage 6: expandable hepatic organoids
To expand the hepatic organoids, they were cultured in an expandable hepatic organoid medium for 3 days.
3) Stage 7: mature hepatic organoids
For differentiation into mature hepatic organoids, the medium was replaced with mature hepatic organoid medium for 6 days. The compositions of all media and small-molecule cocktails for differentiation are detailed in Tables 1 and 2. The equipment is presented in Table 3.
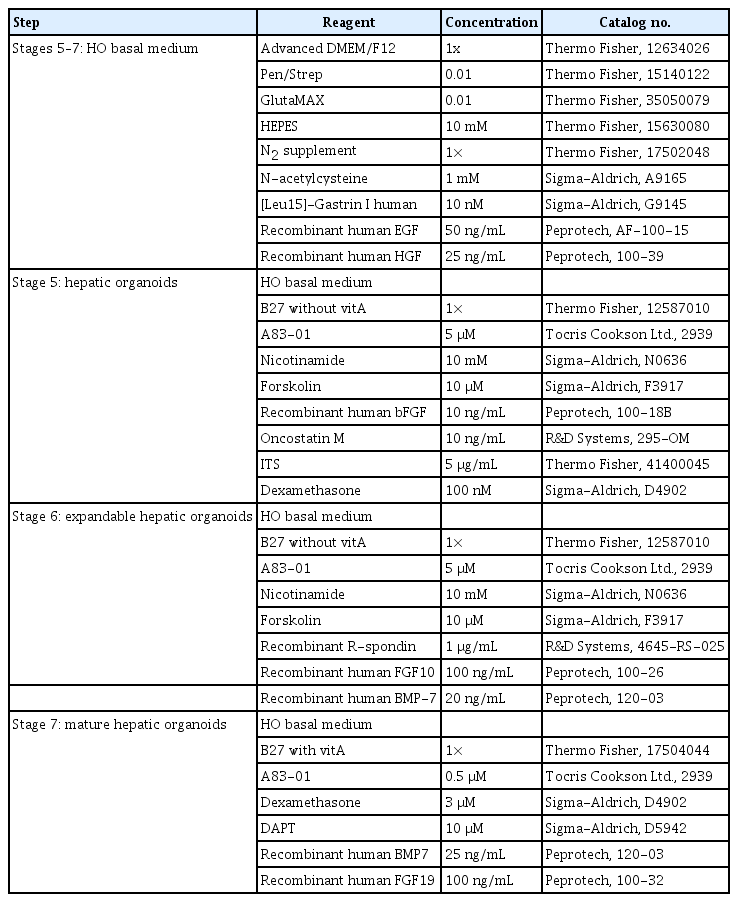
The composition of differentiation media for mature hepatic organoids in the 3-dimensional culture system
Characterization of hepatic-like cells derived from hiPSCs (stages 1–4)
The differentiation of cells derived from hiPSCs was confirmed at each stage (the pluripotent state, hepatic endoderm, hepatic maturation, and hepatic organoids). The pluripotency markers OCT4 and NANOG were expressed in hiPSCs. OCT4 expression significantly decreased in differentiated cells (hepatic endoderm, hepatic maturation, and organoid stages), and NANOG expression also dramatically decreased in each stage compared to hiPSCs. The expression of specific genes at each stage was compared with that in hiPSCs. The expression levels of SOX17 and FOXA2 significantly increased in the definitive endoderm stage and then gradually decreased during differentiation, indicating that hiPSCs are capable of differentiation into hepatocytes (Fig. 2A). The expression levels of both HNF1A and HNF4A were significantly higher in hepatic endodermal cells and in hepatic maturation cells than in hiPSCs, and then dramatically increased in hepatic organoids (Fig. 2A). In addition, hepatic organoids expressed ductal cell markers and bile-duct epithelial cell markers such as SOX9 and CK19 (Fig. 2), suggesting that they could have the properties of hepatic progenitor cells at the onset of liver bud formation (Fig. 2A).

Characterization of the hepatic organoids (HOs) derived from human induced pluripotent stem cells (hiPSCs). (A) mRNA expression of specific markers at each step depending on the differentiation procedure: hiPSCs, hepatic endoderm (HE), hepatic maturation (HM), and HOs. The levels of the pluripotency markers OCT4 and NANOG increased in hiPSCs. SOX17 and FOXA2 expression levels were significantly increased in the HE stage. HNF1A and HNF4A levels significantly increased in the HE, HM, and HO stages, respectively. Expression of the ductal markers SOX9 and CK19 was detected in HOs. (B) Representative images of HOs show expression of LGR5, ALB, and CK19, and Ki-67 positive cells were detected within HOs. All data are presented as means±standard error of the mean. *p<0.05, ***p<0.001 by one-way analysis of variance with the Dunnett multiple comparison test and the two-tailed Student t-test.
The expression levels of the early hepatocyte markers albumin (ALB), transthyretin (TTR), cytokeratin 18 (CK18), and retinol-binding protein 4 (RBP4) significantly increased compared with those of hiPSCs. Moreover, the levels of these early hepatocyte markers gradually increased in the hepatic organoid stage as differentiation progressed (Fig. 2A).
Characterization of mature hepatic organoids (stages 5–7)
After differentiation of the hepatic buds, they were further cultured to differentiate into mature hepatic organoids for more than 10 days. The hepatic organoids were morphologically similar to liver organoids derived from the human adult liver [2,3]. The hepatic organoids could be routinely maintained in hepatic organoid medium. The expandable hepatic organoids enlarged to achieve a spherical cell morphology (Fig. 2B).
We next confirmed the specific markers of hepatocytes (ALB), cholangiocytes (CK19), and adult stem cells (LGR5) in hepatic organoids by immunofluorescence. ALB was highly expressed in most hepatic organoids (Fig. 2B). The expression of CK19 was observed at the edge of hepatic organoids. The percentage of CK19-positive cells was 31.32% in hepatic organoids. Ki-67 positive cells in hepatic organoids were identified to determine whether the organoids were capable of proliferation. We found that about 38.58% of the cells were Ki-67 positive at passage 7 (Fig. 2B). The hepatic organoids remained expandable for at least 3 months. Overall, these findings confirmed that our protocol led to the differentiation of reproductive and efficient hepatic organoids from hiPSCs with our protocol.
The mature hepatic organoids were further characterized using mature hepatocyte markers. The expression of OCT4 and NANOG, which are pluripotency markers, significantly decreased in hepatic organoids, expandable hepatic organoids, and mature hepatic organoids (Fig. 3). We used primary human hepatocytes as a positive control. The mRNA expression of ALB and TTR was significantly higher in mature hepatic organoids and primary human hepatocytes than in hiPSCs (Fig. 3). We examined the CYP family, the members of which are known to play important roles as drug-metabolizing enzymes, such as cytochrome P450-1A2 (CYP1A2) and cytochrome p450-3A4 (CYP3A4). The expression of CYP3A4 and CYP1A2 was significantly enhanced in mature hepatic organoids (Fig. 3). Multidrug resistance-associated protein 2 (MRP2) and bile salt export pump (BSEP) expression levels were significantly higher in mature hepatic organoids than in hepatic organoids (Fig. 3). However, the CK19 level was not meaningfully different between hepatic organoids and mature hepatic organoids (Fig. 3).

Differentiation of mature hepatic organoids (MHOs). Gene expression of human induced pluripotent stem cells (hiPSCs), hepatic organoids (HOs), expandable hepatic organoids (EHOs), and MHOs. ALB, TTR, CYP3A4, CYP1A2, MRP2, and BSEP mRNA levels were significantly higher in MHOs. All data in this figure are presented as the means ± standard error of the means. *p<0.05, **p<0.01, ***p<0.001 by one-way analysis of variance with the Dunnett multiple comparison test and the Tukey test.
To evaluate liver function, we measured the production of ALB, which was significantly higher in mature hepatic organoids than in hepatic organoids (Fig. 4). Similarly, we confirmed that the mature hepatic organoids derived from other hiPSCs (CRL-2097-hiPSC and CMC-hiPSC-009) showed significantly higher ALB secretion than the corresponding hepatic organoids (Fig. 4). Overall, based on liver-specific marker expression and albumin secretion, our results prove that our protocol is sufficiently reproducible to generate mature hepatic organoids and that it is possible to obtain similar results in other hiPSC lines.
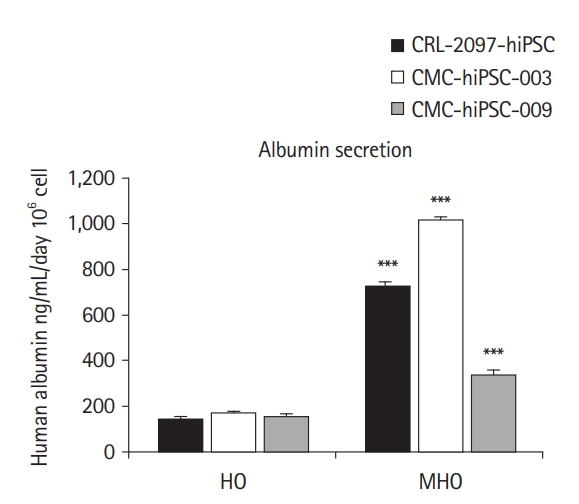
Albumin secretion by mature hepatic organoids (MHOs). The human albumin secretion of MHOs was compared with that of hepatic organoids (HOs) derived from CRL-2097-hiPSC, CMC-hiPSC-003, and CMC-hiPSC-009 cell lines, respectively. Data are presented as means±standard error of the means (n=3) and analyzed by the Student t-test. hiPSC, human induced pluripotent stem cell. ***p<0.001 by the Student t-test.
Discussion
Functional liver organoids are promising cell sources to study a wide range of liver diseases. Currently, many differentiation protocols are available. However, the differentiation of liver organoids from PSCs can fail in some cases, and there is substantial lab-to-lab variation depending on the characteristics of the starting materials. Here, we established a standard operating protocol for a reproducible method to generate functional liver organoids with the combination of 2D and 3D differentiation strategies. To increase the utility of the organoids, through the Korea National Stem Cell Bank [19], we are providing the starting materials together with the protocol for dissemination.
The hiPSC-derived organoids generated with our protocol showed self-renewal ability by acquiring hepatic characteristics during long-term culture in the hepatic organoid stage.
To confirm the presence of hepatic characteristics specific to each differentiation stage, we analyzed the expression of corresponding marker genes. In the fetal liver at 15.5 weeks post-conception, CK19 and SOX9 are used as specific markers of intrahepatic bile ducts and in ductal plates [20]. SOX9 also regulates the development of bile duct morphogenesis in rodents [21,22] and induces expansion in hepatocyte regeneration [23]. In addition, SOX9 influences the differentiation of hepatocytes into bile ductal cells [23]. A previous study showed that the expression of CK19 directly demonstrates connections between cells in the lobule and cells in the bile ducts [20], and during the transition from mature hepatocytes to ductal-like cells [24]. When we used these markers to define the transition of hepatocytes to the intrahepatic bile duct, we found that these markers were more highly expressed in hepatic organoids than in mature hepatocytes. These results indicate that during the hepatic maturation to hepatic organoids, a transition from hepatocytes to ductal cells occurred. In comparison to our previous study [18], the specific genes exhibited similar patterns in the pluripotential, definitive endoderm, hepatic endoderm, and hepatic organoid stages. Furthermore, we reproduced functional and mature hepatic organoids, which had higher expression levels of ALB, TTR, drug-metabolizing enzymes, and transporter genes than found in the hepatic organoid stage. It is also important to measure CYP3A4 activity, ALB production, and the oxygen consumption rate to assess the liver function of mature hepatic organoids [25,26]. CYP3A4 is an essential factor in the metabolic signaling responsible for drug metabolism, as well as the synthesis of cholesterol and various lipids in the liver [27–29]. Higher levels of CYP3A4 gene expression indicate the upregulation of hepatic function in mature hepatic organoids [27,30].
Albumin secretion in mature hepatic organoids dramatically increased compared to the hepatic organoids derived from CMC-hiPSC-003. Furthermore, the mature hepatic organoids derived from other hiPSCs (CRL-2097-hiPSC and CMC-hiPSC-009) also showed significantly increased albumin secretion compared with the corresponding hepatic organoids (Fig. 4). Of particular note, albumin secretion in mature hepatic organoids from the CMC-hiPSC-003 line was far superior to that observed in other mature hepatic organoids. Thus, mature hepatic organoids were also equivalently developed using hiPSCs generated from bone marrow blood cells, as well as fibroblasts.
To apply mature hepatic organoids as a capable hepatic model in a drug screening system, further study will be needed to assess the prospects for research on specific conditions such as severe liver injuries, since the mature hepatic organoids generated in this study did not include hepatic stellate cells, sinusoidal endothelial cells, and Kupffer cells, which are known to cause inflammation and fibrosis [31]. Mature hepatic organoids are currently limited to research on inflammatory signaling and fibrosis occurring in the real human body and require additional injury-induced activation to induce an inflammatory response and fibrosis [32–34]. Therefore, generating a multiple-cell type organoid with an intercellular network will help to extend the potential of research for understanding substantial inflammation pathologies.
In conclusion, using the strategy described in this paper, we successfully reproduced mature hepatic organoids derived from hiPSCs. Through this process, we established a standard operating protocol and provide the starting materials to the public. We expect that the provided protocol and materials can be widely distributed to help many researchers worldwide adopt mature hepatic organoids for specific research purposes, such as screening platforms for the assessment of drug toxicity and drug candidates, chronic disease modeling, and liver toxicity testing, thereby overcoming the current challenges in the generation of mature hepatic organoids.
Materials and Methods
Ethics statement: All experiments using human pluripotnet stem cells were approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (CDC) (approval number 2017-03-07-C-A).
1. Immunocytochemistry
The organoids were fixed for 15 minutes at room temperature with 4% paraformaldehyde, followed by 2 washes in cold PBS to remove the Matrigel. For staining, the organoids were incubated with 0.1% Triton X-100 at room temperature for 10 minutes and then blocked in PBS with 0.1% Tween 20 and 1% bovine serum albumin for 1 hour. The organoids were then incubated in LGR5, ALB, CK19, and KI-67 antibodies overnight at 4°C, followed by incubation with appropriate secondary antibodies for 1 hour at room temperature the next day. Slides were mounted with Faramount Aqueous Mounting Medium (Dako; Cytomation, Glostrup, Denmark). Images were acquired using a confocal laser-scanning microscope (FV3000; Olympus Corp., Tokyo, Japan).
2. Albumin secretion
To quantify albumin secretion by hepatic organoids, cultured medium from each type of hepatic organoid was collected 48 hours after changing the medium. Collected samples were analyzed using a Human Albumin ELISA Kit (E80129; Bethyl Laboratories, Montgomery, TX, USA) following the manufacturer’s instructions. The absorbance was detected by using a Spectra Max M3 microplate reader (Molecular Devices, San Jose, CA, USA) and the results were normalized by the cell number.
3. Reverse transcription-quantitative polymerase chain reaction analysis
Total RNA was isolated from the organoids using a Maxwell RSC Simply RNA Cells kit (Promega, Madison, WI, USA), and cDNA was synthesized by reverse transcription using RNA to cDNA EcoDry Premix (Clontech; Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. Quantitative polymerase chain reaction procedures were run on the QuantStudio 6 Flex system (Applied Biosystems, Foster City, CA, USA) with the PowerUP SYBR Green PCR master mix (Thermo Fisher).
4. Statistical analysis
All statistical analyzes were performed with Prism v.5 (GraphPad Software, San Diego, CA, USA). Data are presented as the mean±standard error of the mean of more than 2 independent experiments. The two-tailed Student t-test was used to compare values between 2 groups. One-way analysis of variance with the Dunnett test was employed to compare values between multiple groups.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by grants from the Korea Centers for Disease Control and Prevention (KCDC 2020-NG-019) (now the Korea Disease Control and Prevention Agency).
Author contributions
Conceptualization: MJK, JL, MJS, JHK; Data curation: MJK, SJM; Formal analysis: MJK, SJM; Funding acquisition: JHK; Methodology: JL, MJS; Project administration: JHK; Resources: JHK; Supervision: JHK; Validation: JHK; Writing-original draft: MJK; Writing-review & editing: JL, MJS, JHK.
Data availability
Please contact the corresponding author for data availability.