Recent advances in liver organoids and their use in in vitro modeling of non-alcoholic fatty liver disease
Article information
Abstract
The liver is involved in physiological activities critical for survival. Chronic liver disease (CLD) frequently progresses to life-threatening liver failure, as evidenced by CLD patients’ high morbidity and mortality rates. Over the years, non-alcoholic fatty liver disease (NAFLD) has received significant attention as an etiology of CLD given its increasing prevalence and progression towards severe pathological conditions such as fibrosis. To answer the urgent need for effective therapeutics that treat CLD, an advanced cellular model, the liver organoid, is used to model and study the complex pathophysiology of NAFLD. Liver organoids recapitulate in vivo aspects of liver tissue such as 3-dimensional cell-cell and cell-extracellular matrix interactions and biomolecular gradients. Moreover, liver organoids can be readily generated from patient-specific liver tissues and induced pluripotent stem cells, enabling their use in a wide range of personalized clinical applications. In recent research, numerous attempts have been made to establish multicellular liver organoids capable of modeling disease phenotypes that involve parenchymal and non-parenchymal liver cells. In this review, we focus on recent advances in liver organoids and highlight the applicability of organoids for modeling NAFLD within the context of cellular sources and composition.
Introduction
The liver serves as a metabolic hub that processes proteins, glucose, lipids, cholesterol, xenobiotics, and therapeutic drugs [1,2]. Each liver lobule, the functional unit of the liver, is composed of multiple spatially organized cell types that include (1) hepatocytes, which are polarized liver parenchymal cells; (2) cholangiocytes, which are epithelial cells lining the bile duct; (3) Kupffer cells, which are liver resident macrophages; (4) hepatic stellate cells (HSCs), which are liver pericytes capable of vitamin A storage and extracellular matrix (ECM) production; and (5) liver sinusoidal endothelial cells (LSECs), which are fenestrated endothelial cells lining the sinusoidal lumen [1,2]. Interactions between these cell types are essential for maintaining normal liver functions [3]. Prolonged liver damage alters cellular interactions, resulting in chronic liver disease (CLD) [4]. Liver fibrosis, an integral component of CLD, is caused by the excessive accumulation of ECM during repetitive injuries and may progress to cirrhosis and hepatocellular carcinoma (HCC) [5,6]. Non-alcoholic fatty liver disease (NAFLD), which is associated with obesity, metabolic diseases, and type 2 diabetes, has recently emerged as a major etiology of CLD. NAFLD encompasses a spectrum of liver disorders characterized by hepatic steatosis that is not due to alcohol consumption. NAFLD may lead to hepatitis, fibrosis, cirrhosis, and HCC. However, effective therapeutic strategies are not yet available due to the complexity of the cellular and molecular pathways that trigger the development and progression of NAFLD. Moreover, the development of new drugs has been hampered by a lack of reliable in vitro models that reflect human NAFLD and fibrosis. In recent research, 3-dimensional (3D) organoids derived from primary tissues or stem cells have emerged as powerful in vitro tools for disease modeling and drug discovery. NAFLD affects 25% of people worldwide and is becoming a global health emergency [6]. In view of these unmet challenges, this review covers the current understanding of NAFLD and highlights recent advances in generating liver organoids to model these diseases.
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
NAFLD
The term NAFLD encompasses two distinct histopathological statuses: NAFLD refers to simple hepatic steatosis without hepatic injury or inflammation, while non-alcoholic steatohepatitis (NASH) refers to steatosis accompanied by hepatocyte ballooning and inflammation either with or without liver fibrosis [7,8]. The pathogenesis of NAFLD without alcohol influence is complex and associated with multiple factors ranging from single genetic mutations to parallel contributions made by interorgan crosstalk [9]. At the cellular level, excess lipid accumulation causes hepatocyte lipotoxicity, which plays a central role in NAFLD initiation and progression [7]. Dietary uptake, genetic mutations, adipose lipolysis, and de novo lipogenesis driven by insulin resistance contribute to the accumulation of free fatty acids (FFAs) in hepatocytes [7]. While steatosis is reversible, persistent FFA accumulation results in dysregulated β-oxidation and elevated levels of toxic lipid metabolites, which cause oxidative stress in the mitochondria, endoplasmic reticula, and lysosomes of hepatocytes [10]. In turn, damaged hepatocytes secrete inflammatory cytokines, chemokines, and damage-associated molecular patterns that activate Kupffer cells, which initiate pro-inflammatory responses and immune cell infiltration that lead to the onset of NASH [10]. LSECs are also involved in different stages of NAFLD progression. LSEC capillarization and dysfunction cause steatosis by stimulating endogenous lipid synthesis and depriving hepatocytes of nitric oxide [11]. LSECs also promote inflammation by overexpressing adhesion molecules, which facilitates the trans-endothelial migration of immune cells [11]. During the pathogenic process, intense pro-fibrotic signals are derived from damaged hepatocytes, Kupffer cells, and capillarized LSECs, leading to HSC activation and fibrosis [11,12]. Overall, NASH is a CLD in which hepatocytes and hepatic non-parenchymal cells (NPCs) influence disease progression by interacting with one another.
Organoids: advent and definition
In vitro 2-dimensional (2D) cell culture has predominated for over a century, providing insights into cell behavior [13]. However, cells that adhere to a flat surface in a monolayer cannot recapitulate the conditions of in vivo cells, which involve 3D cell-cell and cell-ECM interactions and biomolecular gradients established in tissues [14]. To overcome this issue, 3D culture methods that recapitulate in vivo aspects have been developed. The change in dimensionality results in different patterns of cell proliferation, differentiation, apoptosis, and migration [13]. One important component of the 3D culture system is the ECM, which provides integrity to the 3D architecture of cell aggregates, while allowing signaling factors to travel through [15]. Additionally, varying the mechano-chemical properties of the ECM produces distinct signal inputs that impact cell behavior and polarity in a tissue-specific manner [13,15]. Advances in ECM biology, 3D culture techniques, and developmental biology have led to the development of a unique type of 3D culture known as the organoid, which can be used as a disease model with similarities to the in vivo environment [14,16]. Previously, the term “organoid” was used to describe diverse types of 3D culture systems [14], but over the past decade, several groups have proposed a similar but distinct definition with more emphasis on the resemblance to the original organ [17–19]. In this review, we follow the integrated definition of “organoid” proposed by Lancaster and Knoblich [17], Huch and Koo [18], and Clevers [19]: a 3D structure that (1) contains one or more organ-specific cell type, (2) is derived from stem cells or tissue-resident progenitor cells, (3) self-organizes tissue-like structures through cell sorting or spatially restricted lineage commitment, and (4) exhibits functions of the original organ to some extent. Because a strict definition of “organoid” may exclude major progress made with in vitro 3D models of liver diseases, this review also covers recent studies of 3D cell aggregates called “spheroids” based on their similarity to organoids [20].
Liver organoids
Hepatic NPCs and hepatocytes play important roles in the initiation, progression, and manifestation of liver diseases. Therefore, current approaches to the 3D modeling of liver diseases aim to recapitulate critical parenchymal disease phenotypes and non-parenchymal contributions in single organoids composed of multiple hepatic cell types.
1. Primary tissue-derived liver organoids
Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), a Wnt target gene in intestinal crypt stem cells, has been identified as a marker that specifies homeostatic and facultative stem cells in various tissues [21]. A single LGR5-positive adult stem cell from the small-intestinal crypt can generate a self-renewing organoid with the original organ structure and cell composition [22]. This achievement is due in part to the action of the LGR5 ligand R-spondin, which promotes cell expansion by upregulating Wnt/β-catenin signaling [23].
These observations led to the generation of the first liver organoid from damaged mouse liver by culturing biliary LGR5-positive cells in the presence of R-spondin and Matrigel [24]. The resulting cyst-like organoid was capable of long-term clonal expansion and could differentiate into hepatocytes (Fig. 1A). Later, expandable human liver organoids were generated by isolating epithelial cell adhesion molecule (EpCAM)-positive ductal cells from the adult bile duct and embedding them in Matrigel [25]. These organoids had the characteristics of bipotent liver progenitors and could differentiate into hepatocytes. Like intestinal organoids, both types of liver organoids were generated from LGR5-positive or EpCAM-positive ductal cells as an initiating cell source (Fig. 1A, upper) [26,27] and require adding R-spondin-1 and epidermal growth factor to the culture medium [22]. Both methods for generating liver organoids use distinct hepatotropic factors in the expansion and differentiation phases to induce phase-specific characteristics (Fig. 1A). For example, expansion medium (EM) contains soluble factors of the hepatic niche such as hepatocyte growth factor (HGF) [28] and fibroblast growth factor 10 (FGF10) [29] to promote hepatic progenitor proliferation and survival. Additionally, adding high concentrations of the transforming growth factor-β1 signaling inhibitors A8301 [30] and forskolin [31] inhibits the epithelial-mesenchymal transition (EMT) and supports the survival of ductal cells. These combined factors prompt organoids in EM to enlarge continuously across passages as cystic bipotent progenitors that mainly show features of ductal cells rather than hepatocytes. In contrast, differentiation medium contains DAPT [32], HGF [33], dexamethasone [34], FGF19 [35] and bone morphogenetic protein 7 [36], which inhibit biliary fate while promoting hepatocyte differentiation, proliferation, and survival (Fig. 1A). As a result, differentiated organoids exhibit morphology and gene expression patterns similar to hepatocytes and carry out unique hepatic functions such as xenobiotic metabolism, bile acid production, lipid uptake, and glycogen storage [25]. Similar protocols [37] have been used for the large-scale production of liver organoids [38] to investigate the basic mechanisms underlying liver development and regeneration [39] and model liver diseases [40–42].
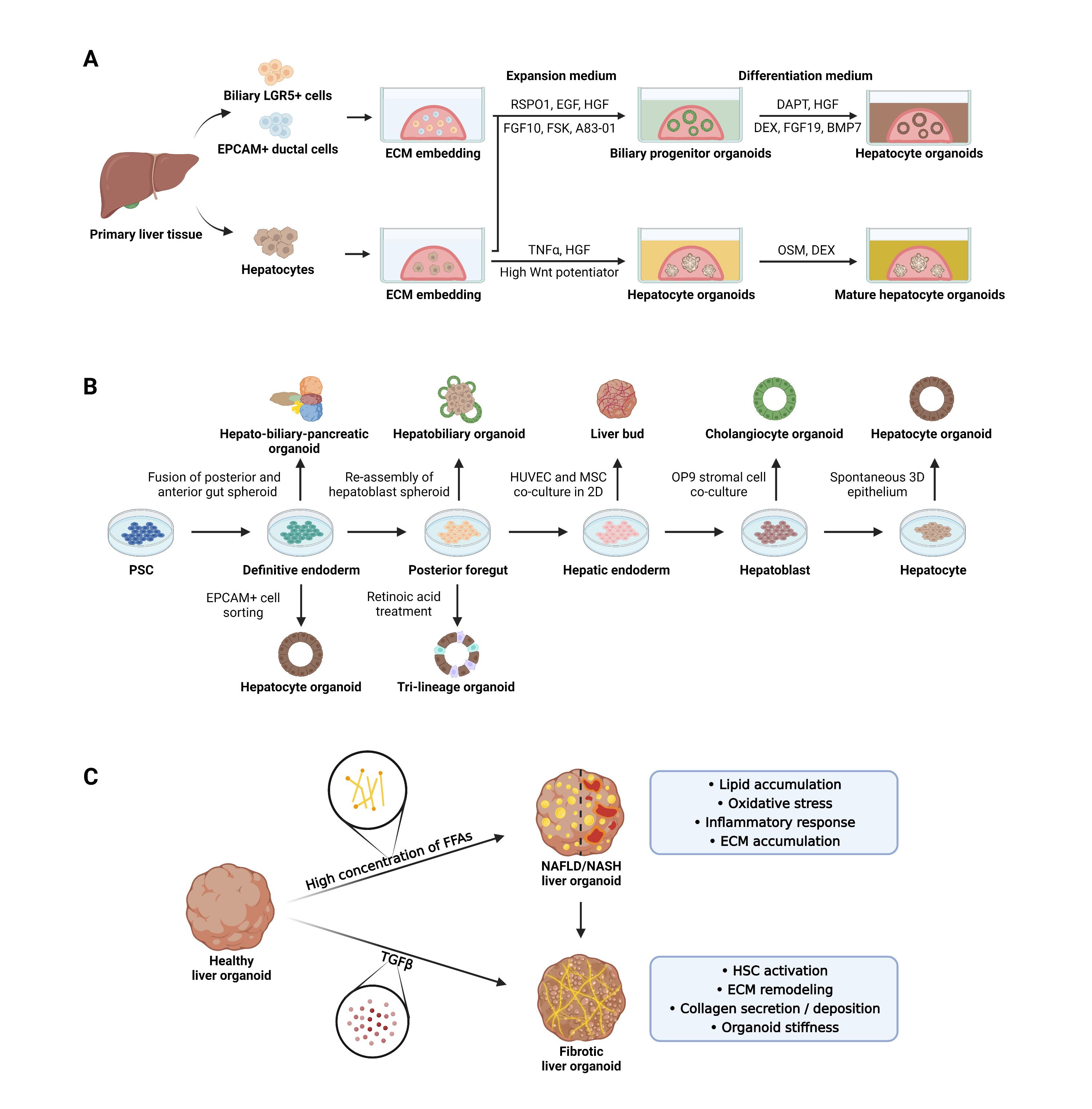
In vitro generation and application of liver organoids to model chronic liver diseases. (A) Liver organoids are generated by embedding biliary leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5)-positive ductal cells, epithelial cell adhesion molecule (EpCAM)-positive ductal cells, or hepatocytes isolated from primary liver tissue in extracellular matrix (ECM). Embedded cells are overlaid with expansion medium containing Wnt potentiators, soluble factors of the hepatic niche, or small molecules that inhibit the epithelial-mesenchymal transition (EMT) and ductal cell survival, resulting in biliary progenitor organoids. Hepatocyte organoids can be generated after transfer to a differentiation medium that promotes hepatocyte differentiation, proliferation, and survival. In other cases, the regenerative capacity of tumor necrosis factor alpha (TNFα) or the action of high Wnt potentiators induce primary hepatocytes to form grape-like hepatocyte organoids that are further matured by the addition of oncostatin M (OSM) or dexamethasone (DEX). (B) Different cell types obtained by conventional 2-dimensional (2D) differentiation of pluripotent stem cells (PSCs) to hepatocytes are used to generate diverse in vitro liver organoid models. Spheroid fusion or re-assembly, co-culture with non-parenchymal cells (NPCs), and the addition of key factors result in the generation of unicellular (cholangiocyte or hepatocyte) or multicellular (hepatobiliary, hepato-biliary-pancreatic, or trilineage) liver organoid models. (C) In vitro non-alcoholic steatohepatitis (NASH) modeling can be achieved by exposing liver organoids to high concentration of free fatty acids (FFAs) and validated by assessing multiple phenotypes of NASH, including lipid accumulation, oxidative stress, inflammatory response, and ECM accumulation. Liver fibrosis can be induced in organoids either by FFA treatment or by exposing them to transforming growth factor beta (TGFβ), which is a potent fibrotic cytokine. BMP7, bone morphogenetic protein 7; EGF, epidermal growth factor; FGF, fibroblast growth factor; FSK, forskolin; HGF, hepatocyte growth factor; HUVEC, human umbilical vein endothelial cell; MSC, mesenchymal stem cell; RSPO1, R-spondin-1; NAFLD, non-alcoholic fatty liver disease; HSC, hepatic stellate cell.
In recent studies, liver organoids have been produced from primary hepatocytes, and these organoids show a distinct morphology compared to biliary progenitor organoids produced from EpCAM-positive ductal cells [43,44] (Fig. 1A, lower). In addition, expandable hepatocyte organoids (Hep-Orgs) have been generated by embedding primary human hepatocytes (PHHs) in Matrigel [43]. Compared to ductal organoid medium, the medium used to produce PHH-derived Hep-Orgs is rich in Wnt potentiators and HGF, both of which contribute to the long-term expansion of organoids. Unlike biliary progenitor organoids, Hep-Orgs do not exhibit a ductal shape; instead, they display “grape-like” structures with multidrug resistance-associated protein 2 (MRP2)-positive bile canaliculi. Furthermore, Hep-Orgs show gene expression patterns and functionality more similar to PHHs than ductal cells. Primary mouse hepatocyte-derived organoids have been generated by harnessing the regenerative capacity of tumor necrosis factor alpha (TNFα) [45] to facilitate hepatocyte expansion in vitro [44]. These organoids expand over 6 months and change the gene expression profile, resulting in enhanced hepatic function in response to changes in medium composition. Interestingly, in contrast with previous findings, frozen EpCAM-negative mature PHHs can produce liver organoids with characteristics similar to biliary progenitor organoids in optimized culture media, suggesting that fluorescence-activated cell sorting of freshly isolated cells is not necessary [46]. These data underline the importance of media composition in organoid expansion and differentiation.
The mechanical aspects of culture conditions have also been investigated using tissue-derived organoids. Comparisons between Matrigel embedding and suspension culture and between static and dynamic cultures in spinner flasks have revealed that physical and mechanical forces impact organoid proliferation and maturation [46,47]. To date, only a few studies have co-cultured tissue-derived organoids with NPCs to generate multicellular liver organoids. Although large 3D aggregates have been produced by the co-culture of 3D hepatocytes and human umbilical vein endothelial cells (HUVECs), no further validation has been conducted in this regard [44]. In a recent study, hepatic progenitor cells and LSECs isolated from mouse liver were assembled to generate vascularized hepatobiliary organoids [48]. The inclusion of LSECs in organoids resulted in enhanced hepatic maturity and viability, the formation of biliary ducts in vitro, and the formation of lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1)-positive vessels upon transplantation. These data indicate the crucial roles of hepatic NPCs in generating liver organoids for modeling liver diseases and obtaining functionally mature cells for regenerative medicine.
PHHs obtained from liver tissues are the gold standard for clinical applications because of their close morphological and biochemical similarity to in vivo hepatocytes [49,50]. However, PHHs rapidly lose their hepatic phenotypes when cultured in vitro as a monolayer [51]. Tissue-derived liver organoids may solve this challenge because they are readily expandable and show comparable maturity to PHHs. However, tissue-derived liver organoids often lack signals from NPCs because epithelial cells are used as initiating cell populations for organoid generation [24,25,37–44,46,47]. In addition, the paucity of donor liver tissues poses a major issue for producing disease-specific or patient-derived organoids. In the future, the above issues can be addressed by (1) enhancing the maturity and expandability of liver organoids and (2) optimizing culture conditions that support the long-term survival and differentiation of both parenchymal cells and NPCs.
2. Pluripotent stem cell-derived liver organoids
Numerous attempts have been made to enhance the maturity of pluripotent stem cell (PSC)-derived 2D hepatocytes by mimicking in vivo liver organogenesis (Fig. 1B) [52–54]. Enhanced hepatic maturity of PSC-derived 2D hepatocytes has been achieved by forming 3D spherical clusters of differentiating cells [55,56]. Based on this finding, conventional 2D-based approaches for the stepwise induction of definitive endoderm, hepatoblasts, and hepatocytes have been adapt to drive self-organizing liver organoid formation [57]. In this section, we review types of PSC-derived organoids and classify them by whether the organoids consist of only epithelial cell types or include NPCs.
Hepatocytes and cholangiocytes are two major epithelial cell types of the liver and originate from common bipotent progenitor cells known as hepatoblasts [58]. Protocols for generating PSC-derived hepatic epithelial organoids often aim to create a self-organized 3D structure from 2D cells at different stages of differentiation, such as definitive endoderm, posterior foregut, hepatic endoderm, hepatoblasts, and even hepatocytes or cholangiocytes after the end of differentiation (Fig. 1B). The resulting organoids are subjected to culture conditions that promote expansion or differentiation towards hepatocytes, cholangiocytes, or both to generate hepatobiliary organoids.
1) Cholangiocytic organoids
The first human PSC (hPSC)-derived cholangiocyte organoid was generated by embedding 2D differentiated cholangiocytes into a mixture of Matrigel and collagen 1 [59]. The organoid exhibited polarized cyst morphology with a luminal space, which later budded into a branching tubular structure. The biliary function of the organoid was assessed by the multidrug resistance protein 1 (MDR1)-mediated efflux of small molecules and bile acid transport [59]. Subsequent studies have generated cholangiocyte organoids by introducing a shift to the 3D culture system at specific stages of 2D hepatic differentiation. For example, co-culture of hPSC-derived hepatoblasts and OP9 stromal cells under 3D conditions produced branching biliary structures with luminal spaces in gels composed of Matrigel and collagen (Fig. 1B) [60]. Under the influence of growth factors and OP9 stromal cell-derived Notch signaling, simple cell aggregates self-organize into cysts and show multiple cholangiocytic functions.
Instead of OP9 cells, a combination of early biliary specification signals including FGF10, retinoic acid (RA), and activin has been used to derive cholangiocyte progenitors from hPSCs [61,62]. These 2D cholangiocyte progenitors were embedded in Matrigel and differentiated into 3D functional cystic organoids that displayed MDR1-mediated transport, bile acid export, and biliary-specific enzyme activities [61]. Of note, both types of organoids produced by OP9 cells and cytokine signals were assessed for their clinical applicability, generating patient-specific cholangiocyte organoids to model key phenotypes of polycystic liver disease and cystic fibrosis-related cholangiopathy.
2) Hepatocellular organoids
Several studies have generated hPSC-derived liver organoids mainly composed of hepatocytes. With methods similar to those used to generate tissue-derived ductal organoids [25], EpCAM-positive cells isolated from induced PSC (iPSC)-derived endoderm produce 3D hepatic progenitor-like organoids in Matrigel [63]. These organoids can be expanded without losing their differentiation capacity and differentiated into more mature types of organoids that show hepatic gene expression and functions with cell polarization. Additionally, patient-derived organoids produced using this method successfully recapitulated the disease phenotypes of citrullinemia type I.
Another recent approach has shown that hPSC-derived 2D cells undergoing hepatic maturation in Matrigel spontaneously give rise to 3D spherical epithelium, closely resembling previous tissue-derived organoids [64]. In a specially designed hepatic medium, 3D organoids showed self-renewal capacity while maintaining key hepatic features essential for assessing in vitro drug toxicity and modeling liver diseases.
3) Hepatobiliary organoids
Because of the bipotentiality of hepatic progenitor cells, shifting cultures of hPSC-derived differentiating cells (presumptive hepatoblasts) from 2D to 3D conditions at the end of the hepatic specification stage leads to expandable hepatic organoids that can differentiate into both hepatocytes and cholangiocytes [65]. Taking advantage of this, several hepatobiliary organoid models that capture the 3D interactions between hepatocytes and cholangiocytes have been developed. The hepatic differentiation of hPSCs by a sequential culture in the presence of a mix of mTeSR and cholesterol promotes mesoendodermal commitment, leading to the formation of hepatobiliary organoids [66]. This method also allows the derivation of Notch-providing endothelial cells, which promote biliary differentiation, and the formation of cholangiocytic cysts near hepatocyte monolayers in organoids. In addition, 3D differentiation of hPSC-derived posterior foregut cells in Matrigel induces a sequential formation of hepatic endoderm spheroids and hepatoblast spheroids, eventually producing hepatic organoids with bile canaliculi systems that can be used to model some features of NASH [67]. In a recent study, the structural and functional complexity of organoid models was enhanced by the generation of hepato-biliary-pancreatic (HBP) organoids from hPSCs. HBP organoids resemble HBP domain explants from embryonic day 10.5 mice (Fig. 1B) [68]. The hPSC-derived HBP organoids were generated without extrinsic signaling factors by fusing CDX2-positive posterior gut spheroids and SOX2-positive anterior gut spheroids, underscoring the importance of adjacent paracrine signaling during organogenesis.
4) Multicellular liver organoids with NPCs
Multiple studies have attempted to develop enhanced organotypic models composed of hepatocytes and multiple NPCs. For example, 2D-cultured HUVECs and mesenchymal stem cells (MSCs) with hPSC-derived hepatic endoderm can self-organize into 3D organoids, named liver bud organoids, capable of vascularization when transplanted in vivo (Fig. 1B) [69]. This unique transition from 2D to 3D structures depends on the contractile force provided by MSCs and matrix stiffness during organoid generation [70]. A subsequent study demonstrated that the HUVECs and MSCs could be substituted with endothelial cells and septum transversum mesenchyme, both of which are derived from iPSCs, suggesting that liver bud organoids can be generated entirely from the human iPSCs of a single donor [71]. This approach is based on cell assembling, in which each cell type is differentiated separately from hPSCs in 2D conditions before being combined for self-organization in 3D conditions. In contrast, a few protocols enable the concomitant specification of multiple cell types in single developing liver organoids. hPSC-derived foregut spheroids can be differentiated into hepatic parenchymal and NPCs in Matrigel in the presence of RA (Fig. 1B) [72]. Multicellular liver organoids are composed of parenchymal populations with a periportal identity and a small population of NPCs that include biliary cells, HSCs, and Kupffer cells as determined by single-cell RNA-sequencing. In particular, the inclusion of Kupffer cells and HSCs enables in vitro modeling of NASH. The liver organoids produced from hPSC-derived foregut cells are also useful for studying drug-induced liver injury (DILI). The liver organoid-based toxicity screening system allows high-speed live imaging in 384 wells and the ability to measure viability and mitochondrial/cholestatic toxicity with high predictive values for multiple drugs at different concentrations [73]. These studies have mainly focused on functional features of organoids related to diseases or DILI. Therefore, the detailed phenotypes, distributions, and functional roles of each type of NPC in organoids need to be explored.
A major benefit of using iPSCs in organoid generation is the unlimited supply of cell sources and their potential to differentiate into multiple liver cell types while retaining donor-specific genetic signatures. Using iPSCs allows continuous non-invasive, patient-specific research and is particularly useful when a single mutation causes abnormalities in multiple cell types [74]. However, hPSC-derived organoids exhibit lower hepatic maturity than PHHs and show uncontrolled variation in the concomitant derivation of NPCs from one another. Addressing these issues and producing uniform liver organoids can minimize data variability when modeling liver diseases and assessing the predictive hepatotoxicity of drugs.
Liver organoids in NAFLD modeling
Organoid-based in vitro modeling of NAFLD can be conducted in a similar way to conventional 2D-based approaches [75,76]. In general, a high concentration of FFAs initiates steatosis, and end points ranging from simple steatosis and inflammation to fibrosis are set for evaluation (Fig. 1C). This review summarizes recent progress in using liver organoids to recapitulate major aspects of NAFLD with different evaluation end points (Table 1) [40–42,64,67,72,77–79].
1. Primary tissue-derived liver organoids in NAFLD modeling
In 2017, hepatic steatosis was modeled in vitro using feline liver organoids generated from biliary duct fragments [40]. FFA treatment caused a darkening of the organoid morphology, as well as an increase in cytoplasmic lipid accumulation and cell death. These FFA-induced phenotypes could be either rescued or aggravated by β-oxidation enhancers or inhibitors. A subsequent study identified two anti-steatosis drug candidates using the same feline liver organoids [77]. These studies reveal the importance of inter-species differences in metabolism-related gene expression between human and feline organoids.
Recently, a clinically relevant study was conducted using liver tissue from mice with methionine/choline-deficient (MCD) diet-induced NASH [41]. Organoids were generated from mice fed a high-fat MCD diet for 4, 8, and 12 weeks, representing the mild, moderate, and severe NASH phenotypes, respectively. Although liver organoids were successfully generated from each group, the moderate and severe NASH groups exhibited delayed generation of smaller spheroid-like structures compared to cystic organoids derived from control mice and mild NASH mice. Multiple features of NASH progression were observed in the NASH organoids, including upregulation of interleukin (IL)-1β, collagen 1, and α-smooth muscle actin, along with signs of EMT. Regardless of NASH severity, amphiregulin (AREG), insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2), and G protein-coupled receptor 137 (GPR137) were upregulated in both NASH organoids and NASH mouse liver tissues, implying their potential as diagnostic biomarkers [41]. More recently, a human-based study was conducted using bipotent ductal organoids derived from the irreversibly damaged livers of NASH patients [42]. Although organoids were successfully derived from all human subjects, NASH patient-derived organoids had a nearly 5-fold poorer organoid-forming efficiency, delayed proliferation, and a lower maximum passage number when compared to healthy patient-derived organoids. Accordingly, transcriptome analysis revealed a downregulation of cell cycle and growth-related pathways in all NASH patient-derived organoids. Several NASH-related genes, including major histocompatibility complex class 2 subunits [80] and aldo-keto reductase family 1 member B10 (AKR1B10) [81] were upregulated in some NASH patient-derived organoids. Furthermore, NASH patient-derived organoids exhibited multiple features of the NASH liver, such as susceptibility to apoptosis and lipid accumulation in response to FFAs [42].
While the above studies used ductal organoids devoid of NPCs, other studies have used multicellular liver organoids assembled from different primary cell sources to model a broader spectrum of NAFLD. Organoids composed of hepatocytes, Kupffer cells, HSCs, and LSECs have been used to investigate the role of miR-122 in NASH and hepatic fibrosis [78]. Consistent with in vivo data, miR-122 inhibition in organoids resulted in histopathology of NASH and fibrosis, including Mallory bodies, hepatocyte ballooning, and collagen deposition. Although no direct comparison was made, different gene expression patterns for inflammation, fibrosis, and insulin resistance were observed in organoids with and without NPCs, implying that NPCs play important roles in modeling liver pathology. An additional type of multicellular organoid has been produced by assembling PHHs and crude fractions of hepatic NPCs from different donors [79]. FFAs were found to induce cytochrome P450 family 2 subfamily E member 1 (CYP2E1) production, lipid accumulation, and pro-fibrotic collagen type I alpha 1 (COL1A1) expression depending on the different donor combinations. Notably, organoids composed of PHHs and NPCs with a patatin-like phospholipase domain-containing protein 3 (PNPLA3) mutation [82,83], which is known to promote NAFLD, exhibited a high baseline fibrotic response in the absence of FFA treatment.
2. PSC-derived liver organoids in NAFLD modeling
hPSCs are useful sources for generating liver organoids to model NAFLD in vitro. A recent study produced hepatic organoids from hPSCs and used them to test the toxicity and efficacy of drugs that target liver steatosis [64]. Similar to NASH livers, the organoids exhibited lipid accumulation and compromised mitochondrial respiration in response to FFA treatment. These phenotypes were significantly reduced by L-carnitine, a β-oxidation enhancer. However, unlike treatment with acetaminophen, carbon tetrachloride, or lipopolysaccharide, FFA treatment failed to induce a prominent inflammatory response, possibly due to the lack of NPCs within the organoids [64]. However, hepatobiliary organoids generated from hPSC-derived hepatoblast spheroids contained distinguishable ductal and hepatic portions and could model multiple features of NAFLD [67]. Upon FFA treatment, dose-dependent increases in lipids, reactive oxygen species, and lipid peroxidation were observed in these organoids. Furthermore, transcriptome analysis revealed that the FFA-treated organoids were similar to NASH liver tissues and different from healthy liver tissues and control untreated organoids [67]. The ductular reaction, a symptom of NAFLD that leads to fibrosis progression, is characterized by the proliferation of reactive bile duct cells in response to liver injuries [84,85]. Like NASH liver tissues, FFA-treated liver organoids exhibited increased biliary gene expression with a concurrent increase in cytokeratin 7-positive biliary cells and a decrease in albumin-positive hepatocytes over time. Moreover, bile canaliculi-related features of NAFLD [86] have been recapitulated in FFA-organoids, as evidenced by the deterioration of the bile canaliculi network and the loss of hepatocyte polarity. A trilineage organoid model that included hepatocytes, Kupffer cells, and HSCs was produced from hPSCs and used to model steatohepatitis [72]. In these organoids, FFA treatment enlarged lipid droplets, caused hepatocyte ballooning, and increased the expression of several inflammatory cytokines, including IL-6, indicating progression towards NASH rather than simple steatosis. Furthermore, upregulation of IL-8 and TNFα was observed in the trilineage organoids but not in spheroids from isolated hepatocytes (E-cadherin-positive) or non-hepatocytes (E-cadherin-negative). In addition, conditioned media from FFA-treated trilineage organoids induced the migration of monocytes in vitro, mirroring the infiltration of immune cells in the NASH liver [72]. These findings indicate the importance of Kupffer cells in liver organoids and highlight the crucial role of the interaction between parenchymal cells and NPCs to induce inflammatory responses in organoids.
Although a few multicellular organoid models have successfully mimicked FFA-induced inflammation and fibrosis, the role of LSECs in organoid-based modeling of NAFLD is yet to be explored. LSECs are the most abundant NPCs in the liver, and they play an important role in the pathogenesis of NAFLD [87]. Therefore, LSECs are essential for using multicellular liver organoids to accurately model NAFLD. Moreover, in previous studies, high doses of FFAs that exceed the human physiological range have been used to induce NAFLD. Because FFAs are a major driver of lipotoxicity, the concentrations, types, and administration methods of FFAs, as well as other contributing molecules such as carbohydrates and lipogenic metabolic regulators, should be taken into account to precisely model NAFLD induction using liver organoids [88,89]. Incorporation of NPCs into liver organoids, combined with precise emulation of a lipotoxic milieu, can allow modeling of a broad spectrum of NAFLD while using a physiological range of pathological stimuli.
Conclusions and future directions
Liver organoids hold great potential as liver disease models because of their physiological relevance, expandability, and applicability in drug screening and cell therapy. However, further improvements of organoid systems are necessary to optimize the use of liver organoids. For example, reproducibility in terms of size and cellular spatial organization must be improved, particularly in multicellular organoids. Structural variation may alter cell-cell interactions and accessibility to pathological stimuli, resulting in variable pathological outcomes between organoids. Moreover, several liver organoid models, particularly those derived from PSCs or ductal tissues, exhibit low hepatic maturity, which may impact disease induction and drug screening. Most importantly, a lack of luminal vascularization results in incomplete disease modeling and limits the even distribution of nutrients and pathological stimuli to the organoid core. The absence of vasculature restricts organoid size to a non-necrotic level, hampering the generation of the complex multicellular structures required for modeling in vivo pathologies. Fortunately, these issues are being addressed by ongoing advances in NPC differentiation protocols and culture techniques. Robust production of authentic NPCs can facilitate the generation of functional multicellular organoids in which endogenous signals from NPCs promote both hepatic maturation and vascularization. In addition, custom-designed scaffolds and ECMs are being developed to support the function and survival of multiple cell components while maintaining the structural homogeneity of multicellular organoids. Organoid cultivation in advanced formats, such as microfluidics or organs-on-a-chip, can also promote maturity, homogeneity, and vascularization. In the future, standardized and homogenous multicellular liver organoids may be used as non-invasive clinical substitutes for conventional invasive, biopsy-based disease evaluation [90]. Overall, liver organoid techniques are evolving, and organoids represent promising tools for patient-specific drug screening, cell replacement, and whole-organ transplantation.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by a National Research Foundation of Korea (NRF) Grant (No. 2018M3A9H1019504) funded by the Ministry of Science & ICT (MSIT), Republic of Korea.
Additional contributions
All figures were created with Biorender.com
Data availability
Please contact the corresponding author for data availability.

