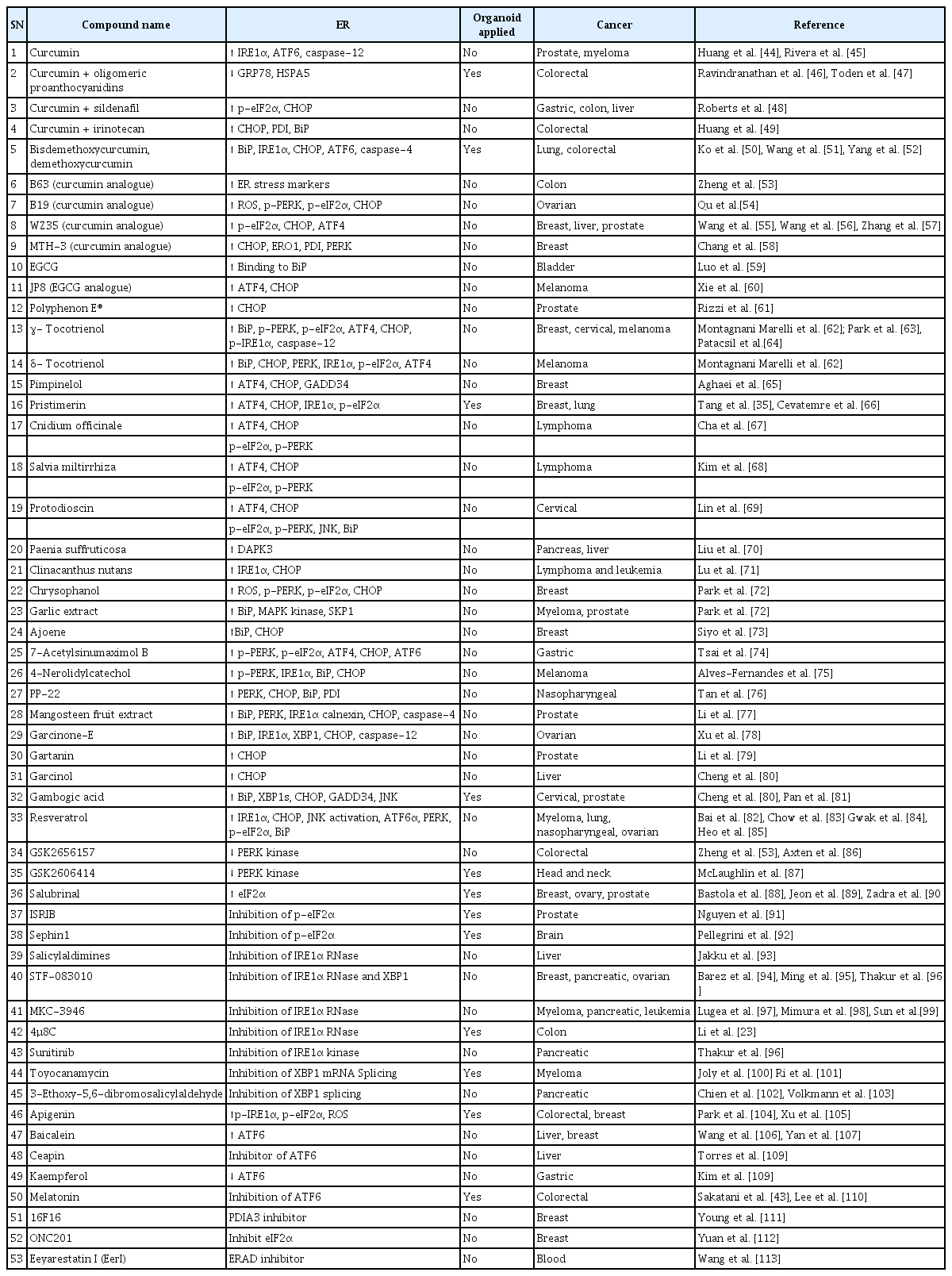
Endoplasmic reticulum stress and organoids
Article information
Abstract
Organoids represent an advanced tool in cell biology and have redefined biomedical research. Organoids are ideal for studies of biological processes, pharmacological studies, and therapeutic research to imitate pathological processes and preserve genetic integrity. The endoplasmic reticulum (ER) is the central organelle responsible for protein folding, post-translational adaptations, and membrane and luminal protein transportation. ER stress is a disorder influenced by a range of physiological and pathological causes, such as nutrient deficiency, impaired glycosylation, calcium depletion, oxidative stress, DNA damage, and energy disruption. Disturbance of the ER environment triggers aggregation of unfolded/misfolded proteins, accelerating ER stress. The unfolded protein response (UPR) is a transduction mechanism that activates cells in response to ER stress to restore ER homeostasis, altering cancer development and progression. However, the mechanisms through which sustained and unresolved UPR signaling triggers a switch from pro-survival to pro-death pathways remain unclear. Immutable and environmental stimuli that modify protein homeostasis are often incorporated into tumor cells, thereby generating ER stress. Herein, we discuss challenges and advances in fundamental and clinical cancer studies on ER stress. Additionally, current trends in organoid technology are summarized to fill the gap in our knowledge of the relationship between cancer and ER stress, with the UPR representing a future tool for investigating drug response screening and potentially revolutionizing the workflow of new cancer drug development.
Introduction
Organoids are characterized by three-dimensional (3D) epithelial primary cell cultures that develop and self-organize from stem cells via cell sorting and spatially restricted descendant commitment, similar to the in vivo process [1]. Embryonic stem cells or induced pluripotent stem cells (known colloquially as pluripotent stem cells or PSCs) and adult stem cells (ASCs) can construct organoid cultures [2]. Organoids in suspensions often exhibit circular or irregular shapes or are embedded in various types of matrices [3]. In vitro organoids have several benefits in human physiological studies and disorder simulations, preclinical studies, and cell culture systems. Furthermore, an organoid can mature and advance in all directions in 3D culture system, enabling the visualization of organ growth and genetic variability in animal models [4]. Different types of growth factors and small molecules are used during the organoid culture process, often in a tissue-specific manner, to control the signaling pathways necessary for organoid self-renewal, proliferation, and differentiation. The first strategy is focused on the division of PSCs, which assemble themselves to form organoids unique to the tissue, such as the optic cup, brain, kidney, prostate, lung, pancreas, and intestine [5-9]. Organoid model-based research for disease analysis is currently being conducted for cancer, malignancy, developmental disabilities, and chronic infectious diseases.
The endoplasmic reticulum (ER) is an organelle closely involved in controlling protein synthesis in the cellular compartment. It detects misfolded proteins and restores protein homeostasis via protein folding. Defective protein folding within the ER and during pathological stress conditions leads to perturbations in ER homeostasis. This imbalance in ER homeostasis activates an ensemble of transcriptional programs known as the unfolded protein response (UPR) [10,11]. Three ER membrane-embedded sensors regulate the UPR: double-stranded RNA-activated protein kinase-like ER kinase (PERK, also known as EIF2aK3), inositol requiring enzyme 1α (IRE1α, also known as ERN1), and activating transcription factor 6 (ATF6). The UPR attenuates protein load on the ER through a temporal shutdown of protein translation along with a complex program of gene transcription mediated by distinct transducers such as ATF4 (for PERK), cleaved ATF6 (for ATF6), and spliced XBP1 (sXBP1; for IRE1α) [12]. These factors directly trigger the transcription of chaperones or proteins participating in redox homeostasis, protein secretion, lipid/fatty acid synthesis, or cell death. However, during ER stress, GRP78 dissociates from each of these sensors, allowing the activation of various adaptive signaling pathways in a collective effort to relieve ER stress [13].
UPR dysfunction is implicated in many human diseases, including cancer, diabetes, neurodegeneration, ischemia, and infectious diseases. Over the past decade, several pieces of evidence have suggested that the three arms of the UPR are highly activated in conditions characteristic of the tumor environment, such as hypoxic, acidic, and nutrient-deprived milieus [14]. Although sustained UPR activation is linked to the induction of apoptotic signaling, cancer cells can bypass this apoptotic switch and exploit the UPR to promote proliferation, metastasis, and chemoresistance in several cancer types. However, unresolved ER stress can lead to excessive UPR activation, which ultimately results in programmed cell death [15].
Thus, dysfunction of the ER stress response has been found to be correlated with various intracellular physiological conditions, and unsurprisingly, with dysregulation of the innate and adaptive immune response. Recent research has shown that UPR sensors control the growth, differentiation, activation, and development of cytokines, as well as apoptosis in several types of immune cells, including T cells, B cells, dendritic cells, macrophages, and myeloid-derived suppressor cells [16]. As such, the emerging role of UPR effectors in the management of a variety of immune disorders, including cancer, has focused researchers’ attention on targeting UPRs [17]. In many cases, ER stress measurements in two-dimensional (2D) cell culture methods may fail to connect with the findings of animal investigations. Moreover, in cancer cells, numerous gene mutations have been found to enhance cancer progression by causing misfolding and accumulation of unfolded proteins in the ER lumen. In these conditions, organoid models will be handy for investigating cancer and ER stress-related diseases. In this paper, we review the significance of organoid research on ER stress regulation, which has yielded several physiological findings of preclinical importance, and describe the potential value of organoids as a method for drug development.
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Applications of organoids to explore cancer and the UPR
Over the past decade, clinical trials in the field of cancer biology have generally relied on 2D cancer cell lines in vitro and on animal models in vivo. These techniques have yielded enormous insights, but after numerous passages, cell lines may not accurately represent the pathophysiology of the original parent tumors [18]. In contrast, organoid technologies make bridges between 2D cell line cultures and in vivo physiological and pathophysiological models, facilitating recapitulation of self-renewal, differentiation, and pathologies responsible for human diseases, including infectious diseases, genetic abnormalities, and cancers [19]. Tumor organoids can now be used to identify core molecular mechanisms of ER stress that may be correlated with cancer cell survival, proliferation, and tumor progression, and facilitate drug testing in vitro. Furthermore, organoids can be engineered with tumorigenic alterations to model cancer initiation, evolution, initiating cells, and oncogenic pathogens; the ability to incorporate these processes is thought to be a closer representation of human disease. In organoid research, stem cells have presented a major breakthrough in terms of understanding cancer progression, drug resistance, and recurrence under different stress conditions, and the stemness of stem cells is regulated by the UPR [20,21].
ER stress has generated immense interest in studying human cancerous organs’ physiology since the UPR plays an essential role in regulating stem cell differentiation, and targeting the UPR may therefore be a therapeutic strategy. UPR is activated in tumor cells to alleviate ER stress by expansion of the ER lumen, transcription of ER-resident chaperones for protein folding, degradation of misfolded/unfolded proteins, and lipid production needed for rapid proliferation. Although these mechanisms allow cells to adapt to exist in an oxygen- and nutrient-deprived environment, chronic or unresolved ER stress can nonetheless lead to apoptosis. The evaluation of the degree and duration of the UPR activation by sensors of ER stress (IRE1α, PERK, and ATF6) seems critical for cell death or survival [22]. However, using tumor organoids could shift this workflow from a linear to high-throughput matrix process. At the same time, IRE1α plays a critical role in colonic tumorigenesis; inhibition of IRE1α expression decreased the stemness of colon cancer stem cells by suppressing the production of β-catenin [23].
Although andrographolide induces ER stress in cancer cells, the activation of IRE1α significantly increased CHOP protein expression and induced apoptosis in colon cancer cell lines through the GRP78/IRE1/XBP1/CHOP signaling pathway (Fig. 1) [22]. The UPR sensor PERK plays roles in cell protection and apoptosis, depending on stress conditions that are difficult to differentiate in terms of cell morphology in 2D cancer cell culture. This difficulty is resolved by organoid technology due to the similarity of organoids to human organ physiology, as confirmed by the finding of a patient response to matched organoid cultures in approximately 90% of cases [24]. Simultaneously, the induction of ER stress in stem cells causes loss of self-renewal and stemness capacity in a PERK-eIF2α-dependent manner. Nonetheless, the inhibition of PERK-eIF2α signaling resulted in stem cell accumulation in organoids [20]. An organoid study of NCI-H660 cells showed that EPHB4 knockdown or inhibition induced immunogenic cell death and increased the phosphorylation of eIF2α and IRE1α, with implications for more accurate prostate cancer therapy [25].
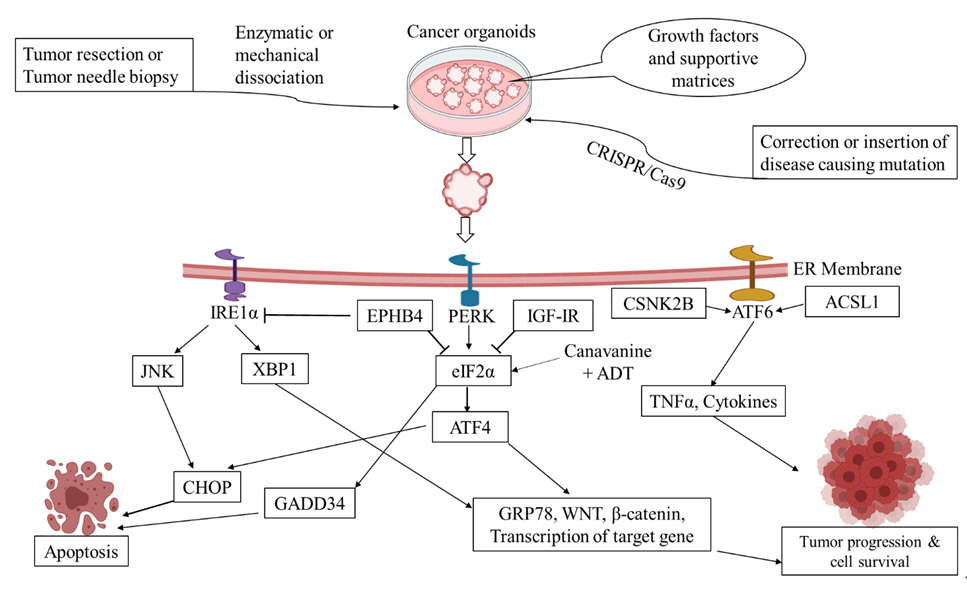
Cancer organoids are developed by genetic mutations, direct tumor resection, or needle biopsy. During ER stress, the accumulation of unfolded proteins in the ER lumen activates PERK, IRE1, and ATF6, stimulating XBP1 splicing and ATF4 and ATF6 translocation in the nucleus, which are responsible for β-catenin and GRP78 upregulation. The transcription of TNFα and other cytokines is regulated by CSNK2B and ACSL1, and Wnt signaling activation is responsible for inflammation, cancer cell survival, and cancer progression. In contrast, IGF-IR, EPHB4 knockdown or inhibition, and ADT with canavanine combination therapy inhibit tumor progression by accelerating the ATF4 and CHOP/ GADD34 pathway.
ER, endoplasmic reticulum;
Similarly, tumor organoids provide a truly unique opportunity to evaluate chemosensitivity differences and to investigate drug resistance mechanisms. For instance, combination therapy of ADT with canavanine triggered catastrophic ER stress via the eIF2α-ATF4(GADD34)-CHOP signaling pathway. Nonetheless, the same arm showed intrinsic radiation insensitivity in response to ADT alone (Fig. 1) [26]. Reduction of IGF-1R function was found to increase cellular stress and cytokine production by phosphorylation of eIF2α to promote an aggressive tumor microenvironment in human breast cancer (Fig. 1) [27]. The role of cancer organoids indicates that ER stress pathways are a novel target for preclinical cancer models and may have value for treatment responses. Moreover, rearrangements in human colonic organoids and the genetic modeling of traditional serrated adenoma through the homozygous loss of APC by the CRISPR-Cas9 technique causes crypt elongation through the Wnt signaling pathway and accumulation of intestinal epithelial stem cells. However, the deletion of Grp78 (either genetically or by small molecule inhibitors of eIF2α kinases) led to complete rescue, implying that the activation of ER stress poses a promising target in APC-deficient colorectal cancers (Fig. 1) [28-30]. In organoid technology, high-throughput genomic technologies have enabled identification of the most frequently mutated genes in cancer models to investigate possible therapeutic interventions [31]. Accordingly, Clarke et al. [32] demonstrated that GRP78 interaction with cleft lip and palate transmembrane 1‐like (CLPTM1L), which is related to ER stress, mediated cytoprotection and chemoresistance in pancreatic adenocarcinoma. Another transcription factor involved in ER stress, ATF6, led to tumor formation in a different organ. Organoid studies have shown that excess and unresolved ER stress in intestinal epithelial cells promoted intestinal inflammation and ulcerative colitis associated with colorectal cancer through activation of ATF6 [33]. As a predictive biomarker, it has been demonstrated that high levels of activated ATF6 increased the expression of TNFα and other inflammatory cytokines in response to tunicamycin treatment in human small intestinal organoids. This led to tumor formation regulated by CSNK2B and ACSL1 [34]. Inhibition of the ATF6 signaling pathway might be a strategy for drug development for inflammatory bowel diseases (Fig. 1).
The unresolved role of ER stress in cancer can be overcome by organoid models
Organoid technology shows considerable promise for improving cancer therapy to restore the impact of cancer stem cells, drug resistance, and drug toxicity, and is also a favorable framework in terms of experimental complexity. Human cell-derived organoid models assist researchers in evaluating the activity of cancer medication in a very simple, but representative way to obtain more information on compound efficacy and potency in the transition from early-stage target identification and characterization to late-stage in vivo research. For example, the precise underlying mechanisms of pristimerin (PRIS), a natural bioactive component, remain very challenging to elucidate in 2D culture. At the same time, patient-derived 3D lung adenocarcinoma cell culture strongly demonstrated that PRIS might induce ER stress-mediated cell death in lung cancer [35]. Furthermore, researchers face many obstacles such as growth methods, physiological accuracy, lack of vascular and neural input, and interstitial pressure while developing organoids (Fig. 2). Despite the hurdles, researchers have developed many organoids derived from normal human tissues and cancer tissues [36].
Cell-cell interactions and cell-matrix interactions change the drug response in vivo. Interestingly, these two phenomena can be conveniently visualized in organoid models, which eventually help researchers develop more specific medication to target a disease of interest [37,38]. For instance, dasatinib showed resistance in recurrent ovarian cancer patients in clinical phase II studies; it appeared to be useful in 2D culture, but the findings in 3D culture resembled those found in patients [39]. Moreover, genetic modifications can be made easily in organoid models, making them more flexible for high-throughput drug screening than other in vitro methods [36,40,41]. In addition, the advancement of imaging techniques for visualizing organoid and protein localization in 3D culture is progressing rapidly (Fig. 2) [42]. Patient-derived organoids have flourished within the framework of personalized medicine [36].
Considering the previous evidence, scientists are becoming more enthusiastic about effective drug development by designing organoid models of ER stress. Sakatani et al. [43] used melatonin, an ATF6 inhibitor, with 5-fluorouracil (5-FU) in a patient-derived colorectal cancer organoid model and reported that melatonin could overcome 5-FU resistance, indicating its anti-cancer potential. Unfortunately, the application of organoid models of ER stress for cancer drug evaluation remains insufficient despite outcomes with promising efficacy (Table 1) [44-113]. The tumor microenvironment influences the induction of ER stress or inhibition of any UPR arm. A previous report stated that the tumor microenvironment contributed to increased UPR signaling in neighboring tissues and promoted tumor development via Wnt signaling [114]. As a result, screening effective UPR-based oncotherapies by applying 2D cell culture models has a high probability of failure for human applications. Instead, down-regulated ER stress sensors have appeared selectively in murine models of prostate tumorigenesis [115]. Consequently, organoid technology may be the only way to address differences in the UPR in cancer growth and animal models.
Organoids in the development of cancer drugs targeting ER stress sensors
The in vitro investigation of drugs using organoids opens up infinite possibilities in biomedical research. Conventionally, cancer cell proliferation, migration, and invasion are investigated via 2D cell culture; however, the 3D organoid model provides an edge to researchers for understanding the cellular and molecular mechanisms in-depth with precision [116,117]. In previous decades, tens of thousands of compounds have been screened prior to clinical trials. Despite rigorous assessments, the majority of these potential drugs (around 80%) fail clinical trials. Effective cancer drugs are even harder to come by, with 95% or more compounds estimated to be unsuccessful at the clinical trial phase [118]. Thus, 3D organoid models are promising for effective screening of drug candidates, and a higher success rate can be expected.
The development of cancer drugs by targeting ER stress and the UPR pathway has become necessary for achieving effective and clinically significant outcomes. Several natural products and chemical moieties have been discovered that target ER stress and UPR arms, and these compounds have been identified as potential treatments in cancer therapy (Table 1) [119,120]. However, very few ER stress-targeting drug candidates have reached clinical trials (ClinicalTrials.gov) [121]. At present, only 12 naturally occurring compounds and chemical moieties have been applied in organoid models. For instance, gambogic acid is a UPR-targeted cancer therapy (Table 1) [122,123]. In 2013, Chi et al. [124] completed a phase II trial in China for advanced malignant tumors, which was not entirely successful. However, Pan et al. [81] have brought this molecule to cancer researchers' attention by applying a pancreatic cancer organoid model, and found that gambogic acid could reduce inhibit the organoid volume. In addition, drug resistance is the foremost hurdle to cancer treatment success, as cancer drugs develop resistance quickly by activating the UPR pathway [125]. For example, PERK-ATF4 activation causes 5-FU resistance in colon cancer [126]. In this case, the use of a PERK inhibitor may solve drug resistance (Table 1). We recommend that GSK2606414 be investigated in human trials for recovering 5-FU resistance in colon cancer because some researchers have shown acceptable results in an ex vivo organoid experiment (Table 1) [87]. In light of the various aspects of drug development targeting ER stress sensors, organoid models could play a significant role in speeding up the discovery and understanding of potential drug candidates.
Conclusions
ER stress and UPR are currently considered the most widely studied targets in the fight against cancer. Cancer cells initiate the UPR and develop drug resistance by activating the UPR sensors ATF6, IRE1α, and PERK and their regulator chaperone proteins. Much remains to be learned about the involvement of UPR in chemotherapy resistance. Nonetheless, it has not been possible heretofore to resolve these issues because of the plethora of conventional 2D cell culture models used to study the UPR and ER stress. Hopefully, patient-derived organoid models will be a blessing for cancer biologists to solve these challenging issues regarding ER stress and the UPR.
Notes
Conflict of interest
Hyung-Ryong Kim has been the president of the Organoid Society since 2018. No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by the Bio & Medical Technology Development Program (NRF-2017M3A9E4047243) of the National Research Foundation of Korea funded by the Ministry of Science and ICT, Republic of Korea.
Data availability
Please contact the corresponding author for data availability.