Generation of human-induced pluripotent stem cell-derived adherent 3-dimensional skin hair-follicle organoids
Article information
Abstract
Background
Regenerating hair follicles (HFs) is a critical medical need for patients who suffer from serious hair loss. To generate equivalent hair-bearing skin systems that could mimic the complexity of native tissues with pluripotent stem cells, floating culture has been employed as a standard method; however, it is still necessary to improve limitations such as the heterogeneity of organoids and the difficulty of handling them, which increases during long-term culture.
Methods
Here, we devise a floating-adherent combinatory culture system to establish skin HF organoids from human-induced pluripotent stem cells (hiPSCs). Specifically, embryoid bodies were generated in a free-floating environment, followed by the induction process, which occurred in adherent conditions.
Results
With our approach, hair germ-like buds were shown to protrude and extend faster. After 100 days of culture, mature cystic skin organoids stratified to form the epidermis, dermis, and outer root sheath, as evident from quantitative polymerase chain reaction and mmunohistochemistry analysis. Dermal condensate cells (Sox2+, PDGFRα+, P75+), which are the precursors of HFs, were detected together with HF stem cells (NFATC1+, LGR5+), putative bulge stem cells (LHX2+, KRT15+) and melanocytes (PMEL+). Notably, our constructed HFs could recapitulate the sensory function of native tissues, as illustrated by the formation of a network of sensory neurons and Schwann cells connecting towards HF cells and epidermal progenitors.
Conclusion
In summary, our results demonstrate a new protocol for the simplified and efficient induction of skin HFs from hiPSCs, thereby contributing to research on optimizing HF growth and investigating novel therapeutic strategies to treat alopecia.
Introduction
The skin is the largest organ in the body, and it plays an essential role in regulating homeostasis and defending against pathogens. Disruption of this crucial barrier results in serious problems that significantly impair life. This complex two-layer organ, which consists of the epidermis (epithelial cells) and dermis (mesenchymal cells) from varied origins [1], is accompanied by appendages [2]. One of the major skin appendages is hair follicles (HFs), which are crucial for physical protection, thermal insulation, and sensory perception [3]. The formation of appendages in general—and hair in particular—strongly necessitates coordinated epithelial–mesenchymal interactions [3]. At a basic level, hair-associated stem cells include melanocyte stem cells (from the neural crest) and HF stem cells (from the ectodermal epithelium) that help maintain the regeneration cycle of hair [4].
Over the past 50 years, the ability to generate human skin models has greatly diminished the use of animals and mitigated the burden of skin diseases by offering an alternative skin source [5–7]. In the literature, although human skin tissue has been reconstituted via various cell-mediated methods [8–10], the resulting systems typically failed to mimic the full complexity of native skin. To establish a skin model with HFs, strategies focused on co-culturing pluripotent stem cell-derived epidermal and dermal cells, or inducing HFs from dermal papilla cells [9,11,12]. However, the reconstructed human HF-bearing skin yielded from these systems was unable to mimic in vivo skin tissues as well as HFs in terms of the supportive neuronal network and pigmentation. In this context, 3-dimensional (3D) organoid cultures have emerged as a potential strategy that exquisitely stimulates the architecture and function of native organs [13,14]. Up to present, feasible protocols to derive skin HF organoids from human-induced pluripotent stem cells (hiPSCs) or embryonic stem cells employed free-floating cultivation [15–17], which provides a more natural environment for the cells [18]. However, it remained unclear whether this method could address the major challenge, which is the ability to maintain skin appendages in long-term cell culture [19]. In the literature, HF induction in vitro was promoted via adherent cultivation, which successfully reconstructed HFs for hair formation [20,21]. However, the efficiency of combining free-floating and adherent culture in reconstructing 3D skin HFs organoids remained uncertain. For that reason, this study developed a novel approach combining floating and adherent cultivation to establish 3D skin HFs that recapitulate in vivo tissues in an attempt to simplify the culture process.
Producing a skin construct through organoid culture holds the potential for enabling feasible cell therapy targeting diseases that cause hair-related problems, such as baldness and, more seriously, alopecia. Although hair transplantation has been applied as an instant remedy, transplanted hair is not maintained in the long term. Furthermore, clinical drugs targeting these conditions still have certain side effects and fail to satisfy the patient’s needs for preventing hair loss rather than rebuilding lost hair [3,22,23]. A model of HFs in skin organoids originating from hiPSCs, therefore, would supply a rich source of native organ-like skin tissue for drug screening for skin disorders and alopecia. Furthermore, this alternative source of HFs, by supplying an unlimited number of samples, would facilitate the optimization of HF maturation, along with discoveries of novel stem cell therapies for hair loss, and advance our understanding of the signaling pathways that govern the higher-order development of skin and HFs.
In this research, we present a long-term culture system that combines floating and adherent cultivation to reconstruct skin and HFs from hiPSCs. We report that skin organoids of hiPSC origin recapitulate key morphological features of the native skin and have the potential to generate de novo HFs. Our method is a novel simplified approach for the feasible reconstruction of skin HF organoids in vitro.
Materials and Methods
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
1. Human-induced pluripotent stem cell lines and culture
Culture experiments were performed with the hiPSC line CMC-003, donated from the National Stem Cell Bank of Korea (Korea National Institute of Health), and originally provided by The Catholic University of Korea. Pluripotent stem cells were cultured on six-well plates coated with 0.5% Matrigel (Corning, NY, USA) and maintained in mTeSR-1 Basal Medium (StemCell Technologies Inc., Vancouver, BC, Canada) with mTeSR supplement (StemCell Technologies Inc.). The medium was replenished every day, and the CMC-hiPSC-003 cells were passaged as tiny clusters at around 40% to 60% confluency (generally every 3 to 4 days) using Gentle Cell Dissociation Reagent (StemCell Technology Inc.).
2. Optimized differentiation of human-induced pluripotent stem cell
For differentiation, colonies of hiPSCs were detached from the culture dish using 10% dispase (StemCell Technologies Inc.) to generate embryo bodies. After dissociation, cells were collected as tiny clusters in E8 medium containing 10 μM Y27632. The appropriate number of live cells needed for an experiment was transferred into an E8 medium containing 20 μM Y27632 in Primaria cell culture dishes (Corning) and incubated at 37°C under 5% CO2 for 48 hours. We designated this time point as day-2. After 24 hours of incubation (day-1), 8 mL of fresh E8 medium was added to each dish to promote cell proliferation and aggregate growth. On day 0, to start differentiation, all cell aggregates were individually collected and transferred to new dishes with 3 mL of E6-based differentiation medium containing 2% Matrigel, 10 μM SB431542 (Tocris, Bristol, UK), 4 ng/mL basic fibroblast growth factor (bFGF; R&D Systems, Minneapolis, MN, USA) and 2.5 ng/mL BMP4 (PeproTech, East Windsor, NJ, USA) to initiate non-neural ectoderm formation. On day 3 of differentiation, to induce cranial neural crest (CNC) cell formation, 200 ng/mL LDN-193189 (LDN) (a BMP inhibitor; Sigma Aldrich, St. Louis, MO, USA) and 50 μg/mL bFGF were added in a volume of 1.2 mL, thus making the final volume 3 mL per dish. On day 6 of differentiation, 1.8 mL of fresh E6 medium was added, bringing the final volume to 4.8 mL. Half of the medium was changed (removal of 2.4 mL of spent medium and addition of 2.4 mL of fresh E6 medium) on days 8 and 10. On day 12, to induce self-assembly of the epidermis, all aggregates were transferred into low-attachment plates (Thermo Fisher Scientific, Waltham, MA, USA) in 4.8 mL of organoid maturation medium (OMM) containing 0.5% Matrigel. OMM is composed of advanced Dulbecco’s modified Eagle medium: nutrient mixture F-12 (DMEM/F-12; Gibco, Waltham, MA, USA) and neurobasal medium (Gibco) at a 1:1 ratio, 0.5× B-27 minus vitamin A (Gibco) and 0.5×N-2 (Gibco) supplements, 0.1 mM 2-mercaptoethanol (Gibco) and 100 μg/mL Normocin (InvivoGen, San Diego, CA, USA). On differentiation day 12, half of the spent medium was replenished (removal of 2.4 mL of spent medium and addition of 2.4 mL of fresh medium) with OMM containing 0.5% Matrigel until day 46. On day 47, the whole medium was replenished with fresh OMM without Matrigel. Starting from day 50 (or longer), half of the medium was replenished (removal of 2.4 mL of spent medium and addition of 2.4 mL) with OMM every 3 days.
3. RNA extraction and quantitative reverse-transcription polymerase chain reaction analysis
Total RNA from organoids was extracted using the TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. The extracted RNA of all samples was stored at -20°C until quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed. The iScript Reverse Transcription Supermix for qRT-PCR (iScript RT Supermix; Bio-Rad, Hercules, CA, USA) was used to synthesize cDNA from total RNA using a Master cycler (Eppendorf, Hamburg, Germany). The concentration of RNA was measured using a Nano Drop spectrophotometer (Thermo Fisher Scientific). Primers were designed using Primer-BLAST (NCBI, https://www.ncbi.nlm.nih.gov/tools/primer-blast). The primer sequences for quantitative PCR are as follows: Lhx2, forward: 5’-GTGGACAAGTCGACAGACGC-3’, reverse: 5’-AGGGTGGGGCTAGTCAAGTC-3’; Pcad, forward: 5’-TCTCGCGTCTCTCCTCCTTC-3’, reverse: 5’-CGCCTCCAAGGTCACTTCAG-3’; Edar, forward: 5’-GCTACCAGATATGCAGGCGT-3’, reverse: 5’-ATGTAGTAGCCAGGGAGGCA-3’; Pdgfrα, forward: 5’-CTGCCTGACATTGACCCTGT-3’, reverse: 5’-CTGCTGGAACCCGTCTCAAT-3’; Ngfr(p75), forward: 5’-AGATCCCTGGCCGTTGGATT-3’, reverse: 5’-AGGTCTTGTTCTGGAGGTGC-3’; Sox2, forward: 5’-TACAGCATGTCCTACTCGCAG-3’, reverse: 5’-GAGGAAGAGGTAACCACAGGG-3’; KRT5, forward: 5’-TCTTGCCGGAGGTAGCAGTG-3’, reverse: 5’-CTGCTTGCACTGAAGCCAGAG-3’; KRT15, forward: 5’-ATGGAGGCTCAGAACCAGGA-3’, reverse: 5’-TCCTGAAGAGGCTTCCCTGA-3’. qRT-PCR was carried out using the StepOne Real-Time PCR System (Thermo Fisher Scientific) with the iTaq Universal SYBR Green Master Mix (Bio-Rad). The conditions were 95°C for 10 minutes followed by 50 cycles at 95°C for 15 s and 60°C for 3 s. Triplicate reactions were carried out for each sample. Gene expression levels were compared statistically using the one-way analysis of variance (ANOVA) (the Kruskal-Wallis test) to quantify differences between experimental groups. p-values <0.05 were considered significant.
4. Immunohistochemistry
In brief, samples were fixed in 4% (v/v) paraformaldehyde overnight at 4°C and then washed in phosphate-buffered saline (PBS). After the tissues were embedded in paraffin, they were cut into 4-μm-thick sections. For immunohistochemical staining, the fixed samples were deparaffinized and antigen retrieval was performed. All samples were blocked in 5% bovine serum albumin (BSA) for 30 minutes and then incubated with primary antibodies overnight at a 4°C refrigerator. The next day, the slides were then incubated with fluorescently labeled secondary antibodies in 1% BSA for 1 hour at room temperature after 3 PBS washes for 10 minutes and counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were obtained using confocal microscopy (K1 Fluo; Nanoscope Systems Inc., Daejeon, Korea). We sectioned 5 to 6 organoids on day 110 for immunohistochemistry (IHC) analysis. All images are representative of samples obtained from 4 separate experiments.
5. Antibodies and reagents
The antibodies used in this study were CD49f (mouse, ab20142; Abcam Inc., Cambridge, MA, USA,), cytokeratin 5 (KRT5) (mouse, ab259429; Abcam Inc.), cytokeratin 15 (KRT15) (rabbit, ab52816; Abcam Inc.), cytokeratin 17 (KRT17) (mouse, ab212553; Abcam Inc.), loricrin (rabbit, ab85679; Abcam Inc.), S100β (mouse, ab11178; Abcam Inc.), SOX2 (rabbit, ab97959; Abcam Inc.), tubulin β-3 (Tuj1) (rabbit, B240864; BioLegend, San Diego, CA, USA) and premelanosome protein (PMEL) (rabbit, ab137078; Abcam Inc.), and Ki-67 (mouse, ab15580; Abcam Inc.).
6. Statistics and reproducibility
The organoid specimens used for histology and qRT-PCR analysis were selected at random and not screened for quality or specific morphological characteristics. Each experiment was performed 4 times (n=4). All statistics were performed using Excel statistical tools or Prism 5 (GraphPad Software, San Diego, CA, USA). Data are reported as the mean±standard deviation. The statistical significance of differences between groups was analyzed using one-way ANOVA (the Kruskal-Wallis test). A p-value less than 0.05 was considered significant: *p<0.05, **p<0.01, ***p<0.001.
Results
1. Morphology of human 3D HF skin organoids
To induce 3D HF skin organoids, we sought to guide hiPSCs toward the surface ectoderm lineage. hiPSCs of the CMC-003 cell line were detached into small pieces of cell clumps and plated on ultra-low attachment plates to create embryoid bodies. We then transferred the embryoid bodies to new plates containing a differentiation medium with key factors, namely bone morphogenetic protein 4 (BMP4) and a transforming growth factor-beta inhibitor (SB431542), to promote epidermal induction (Fig. 1A). This differentiation strategy produced uniform epithelial cysts with a diameter ranging from 500 to 1,000 μm [16]. Next, co-treatment of bFGF and a BMP inhibitor (LDN) was delivered, facilitating the induction of CNC-like cells that give rise to mesenchymal cells. As described elsewhere [16], by day 16, the organoids had an exterior layer of CNC-like cells surrounding the epithelial cells. Remarkably, from day 18, the organoids experienced bipolarization, with an emerging asymmetric structure with a head (the pole composed of epidermal cyst) and tail (the opposite pole characterized by an opaque cell mass) [16]. By day 30, lipid-rich regions emerged in epithelial cysts (Fig. 1B). With extended culture, the tail structure shrank gradually and disappeared in some organoids, eventually forming a matured structure of skin organoids that comprised different cell types. Theoretically, the development of human HFs initiates around weeks 8-10 of gestation; therefore, the culture period was prolonged, allowing the skin construct to form hair bud structures. By day 60, hair-germ-like buds protruded radially on the surface of attached organoids. On day 110 of culture, these hair placode germs significantly extended.
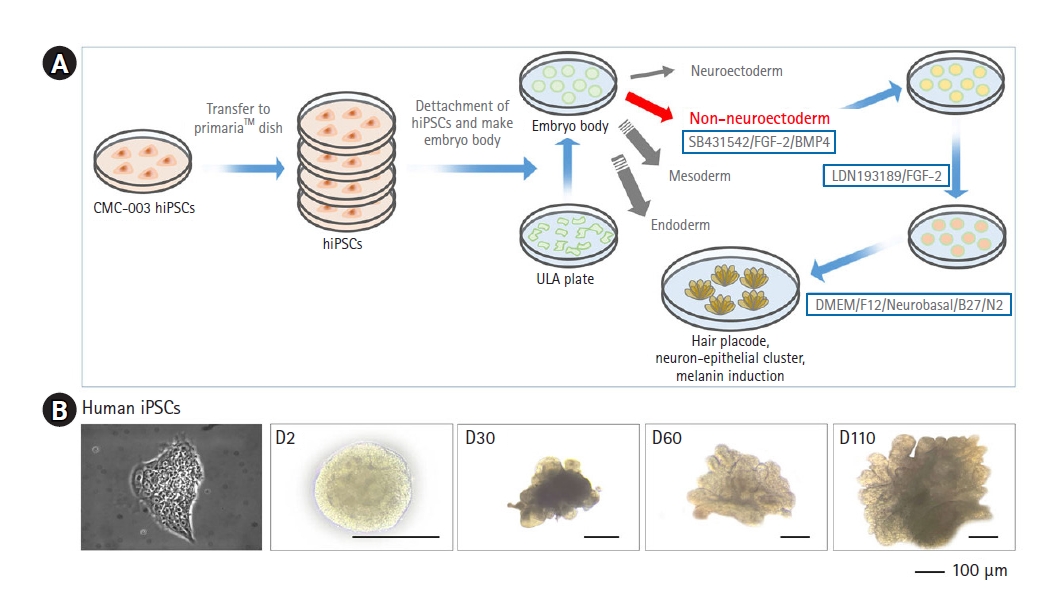
Overview of skin organoid differentiation. (A) Schematic overview of the skin organoid protocol. After the detachment of human-induced pluripotent stem cells (hiPSCs), cells were plated on new plates with a differentiation medium to form embryoid bodies. (B) Comparison of the timeline of skin development. Skin organoids on different days of differentiation. hiPSC under a microscope. On day 2 of differentiation, skin organoid showed sphere-like morphology. Cysts on the skin organoids became visible by day 30 of the differentiation. By day 60 of differentiation, the skin organoids maintained a cystic morphology. Optimization experiments were repeated at least 3 times independently. D, day of organoid maturation; ULA, ultra-low attachment.
2. 3D HF skin organoids comprising stratified specific layers of skin tissue
To confirm whether our HF skin organoids morphologically resembled in vivo tissue, we assessed the mRNA expression of different markers through qRT-PCR analysis. The results showed that organoids with protruding hair placodes expressed epidermal germ cell markers, including LIM homeobox protein 2 (LHX2), P-cadherin (PCAD), and ectodysplasin A receptor (EDAR) (Fig. 2B). The expression level of LHX2 and EDAR, which was similar in the hiPSC and embryoid body day 5 (EB5) stages, significantly increased at day 100. Meanwhile, the PCAD expression level at EB100, which was relatively similar to that of EB5, was significantly reduced in comparison with the initial stage. In addition, a significantly increased level of platelet-derived growth factor receptor alpha (PDGFRα) and the neuroglia-associated marker NGFR (P75) was detected in embryoid bodies by day 100, proving the formation of dermis (Fig. 2C). The high transcription of the marker genes of interest within the constructed skin organoids demonstrated the results of stratification during this period, which constituted the fundamental layers of the skin.

Expression of selected epidermal and dermis markers in quantitative reverse-transcription polymerase chain reaction. (A) Schematic view of dermal condensates of skin organoids. The epidermis and dermis differentiated in the skin organoids, and cysts were formed by day 30. (B) Relative expression patterns of selected epidermal germ cell markers (LHX2, PCAD, and EDAR) in human-induced pluripotent stem cells (hiPSCs) and skin organoids on days 5 and 100 (n=4). (C) The expression pattern of selected dermis cell markers (PDGFRα, NGFR(p75), and SOX2) in hiPSCs and skin organoids on days 5 and 100 (n=4). (B, C) Data are presented as mean±standard deviation. Analysis of variance (the Kruskal-Wallis test) was performed to calculate significance (*p<0.05, **p<0.01, ***p<0.001). D, day of organoid maturation; EB, embryoid body (day).
3. Mature skin organoid carries prominent physiological features of HFs
To further demonstrate whether the 3D organoids resembled and mimicked skin maturation and HF formation in native tissues, we confirmed their architecture based on the expression of key proteins. Specifically, KRT5 (a basal cell marker) and KRT15 (a peridermal marker) were highly expressed on EB100 (Fig. 3A), which was also revealed in IHC analysis results (Fig. 3B). Additionally, the formation of the basal layer and granular layer of epidermis was demonstrated, respectively characterized by CD49F and loricrin as markers (Fig. 3C). Regarding HFs, a growth in the bulge stem cells and outer root sheath in our skin organoids was proven by the elevated level of KRT15 by day 100 (Fig. 3A). In additions, qRT-PCR analysis suggested a remarkably enhanced level of Sox2 transcription at the mature stage, indicating the presence of dermal condensate and dermal papilla cells (Fig. 3D). These cells were shown to accompany the periderm-like layers (KRT5+), immunolabeled by the co-expression of Sox2 (Fig. 3E). Furthermore, we observed that this transcription factor interwove with KRT17+ cells (Fig. 3F), demonstrating that HF precursors were distributed in proximity to the epithelium and HFs. Together, these results demonstrated that the skin organoids were a facsimile of native skin tissue in terms of higher-order skin morphogenesis and HF formation during the maturation process.
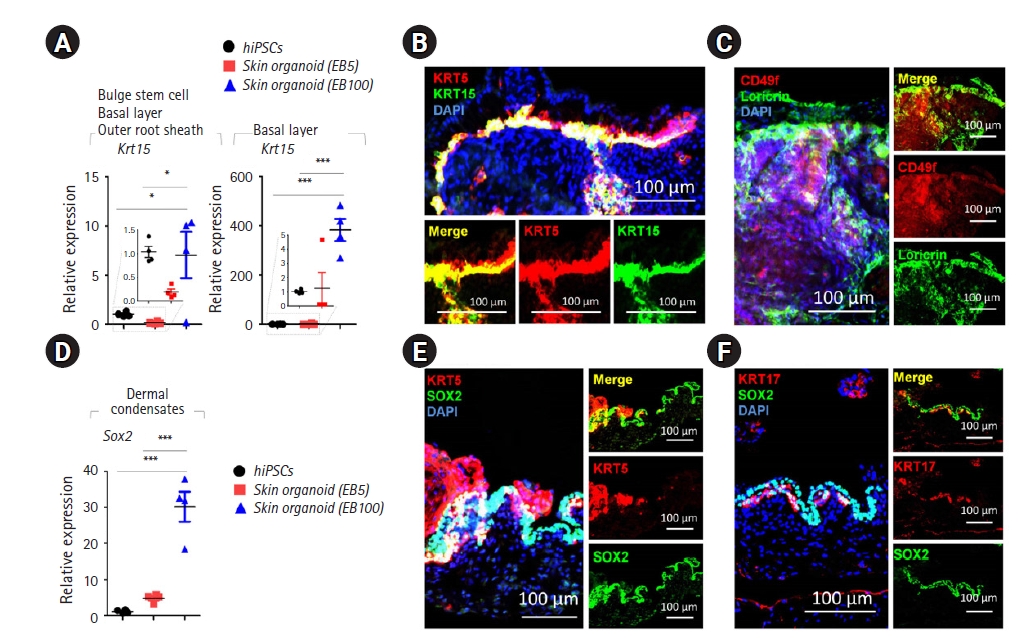
Expression of selected markers in human-induced pluripotent stem cells (hiPSCs) and skin organoids and quantitative reverse transcription polymerase chain reaction (n=4). (A) Relative gene expression patterns of selected bulge stem cell, basal layer, and outer root sheath cell markers (KRT15, KRT5) between hiPSCs and organoids on days 5 and 100. Data are presented as mean±standard deviation (SD). Analysis of variance (the Kruskal-Wallis test) was performed to calculate significance (*p<0.05, ***p<0.001). (B) A day-100 skin organoid was immunostained for KRT5 and KRT15. The KRT5 antibody highlights the epidermis and the outer root sheath of the HFs. KRT15 labels the peridermal layer (scale bar: 100 µm). (C) Immunostaining of CD49f and loricrin. CD49f represents the basal layer, and loricrin indicates the granular layer of the day-100 skin organoid (scale bar: 100 µm). (D) Relative gene expression pattern of dermal condensates (SOX2) in hiPSCs and skin organoids on days 5 and 100. Data are presented as mean±SD. Analysis of variance (the Kruskal-Wallis test) was performed to calculate significance (***p<0.001). (E) Immunostaining of KRT5 and SOX2. The KRT5 antibody labels the epidermis, whereas SOX2 marks dermal condensate cells, dermal papilla cells, and is also expressed in some Merkel cells and melanocytes (scale bar: 100 µm). (F) Immunostaining of KRT17 and SOX2. The KRT17 antibody labels the epithelium and hair follicles (scale bar: 100 µm). EB, embryoid body (day).
4. HFs within mature skin organoids that morphologically recapitulated native tissue
To assess the formation of HFs within the generated 3D skin organoids, we examined the construction of prominent cells and the neuron network. Specifically, we identified the proliferation of HF stem cells on EB100, as evident from the accelerated level of transcription of nuclear factor of activated T cells 1 (NFATC1) and leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), compared to hiPSCs and EB5 (Fig. 4A). The development of the Merkel cell population, indicated by a significantly increased level of KRT20 transcripts, were also detected in mature skin organoids (Fig. 4B). Since organoid HFs are pigmented [17], we assessed the growth of melanocytes through the expression of pattern of selected melanocytes (PMEL). qRT-PCR analysis of organoids with protruding placodes showed a significant increase in the PMEL level by day 100 (Fig. 4C). The result was confirmed by IHC, as PMEL and Ki-67 were expressed highly with a similar pattern (Fig. 4D), implying the proliferation of pigmented cells. The results, hence, suggested that HF skin organoids can recapitulate the skin in terms of pigmentation.

Immunofluorescence analysis of key protein markers in day-110 skin organoids (n=4). (A) Relative gene expression pattern of selected hair follicle stem cells (NFATC1 and LGR5). (B) Relative gene expression patterns of selected Merkel cells (KRT20). (C) Relative gene expression pattern of selected melanocytes (PMEL). (A–C) Data are presented as mean±standard deviation. Analysis of variance (the Kruskal-Wallis test) was performed to calculate significance (*p<0.05, ***p<0.001). (D) Immunostaining of KRT5 and SOX2. KRT17 antibody labels basal and peridermal keratinocytes and hair follicles (scale bar: 100 µm). (E) Immunostaining of a skin organoid shows the distribution of TUJ1 and KRT17 expression, representing melanocytes in the hair follicles and neurons (scale bar: 100 µm). (F) Immunostaining of KRT5 and S100β. The S100β antibody labels satellite glial and Schwann cells (scale bar: 100 µm). (G) Schematic view of distribution of cell clusters (color-coded) consisting of epidermal cells, dermal cells, melanocytes, hair follicle stem cells, neurons, and Merkel cells during skin maturation from day 1 to over day 80 in a skin organoid during maturation. hiPSC, human-induced pluripotent stem cell; EB, embryoid body (day).
Next, the contact of constituted HFs to neuronal cells was considered. We observed that early neurons were characterized by Tuj1-wrapped HFs (KRT17+) and contacted the surrounding epithelial cells (KRT17+) (Fig. 4E). Here, a neuron cell body was clearly exhibited, accompanied by the scattered distribution of a neuron network over the surrounding region that enhanced the connection to the HF. Furthermore, staining results revealed that satellite glial and Schwann cells (S100β+) were distributed surrounding the outer root sheath of the HF (KRT15+) and elongated to their ends, thereby extending their connection (Fig. 4F). Concurrently, the distribution of these sensory cells overlapped the region of epithelial cells (KRT15+) (Fig. 4F). In summary, the data suggested that our skin organoids, with the formation of precursor and pigment cells accompanied by a sensory neuron network, were sufficient to physiologically recapitulate native human skin tissue.
Discussion
The skin, a complex tissue with multilayered structure accompanied by HFs and glands, plays a crucial role in temperature regulation, fluid retention, and sensation mediation of the body [5,17]. Since it is challenging to reconstitute this tissue once damaged, reconstructing appendage-bearing skin in vivo is critical. HF generation has been attempted in previous research [24,25], but only preliminary results were achieved for constructing HFs within skin in mice [17]. Here we devised a floating and adherent combined culture system that generated 3D HF skin organoids from hiPSCs. Complexes of hair placodes and neuron-epithelial clusters capable of inducing melanin were obtained after 100 days. The produced HF skin organoids were adherent to the culture dish and composed of stratified epidermis, dermis, and dermal condensates, the key component carrying hair inductive potential and regulating the hair cycle [1]. HFs were formed and pigmented, equipped with a network of sensory neurons and Schwann cells, demonstrating that our skin organoids could recapitulate key aspects of human skin tissues.
The organ-inductive potential of stem cells exists only during organogenesis, then is maintained in the stem cell niche of each organ that is responsible for tissue repair after birth [26,27]. For hair, inductive epithelial and mesenchymal stem cells provide differentiated HF cells, thereby enabling the occurrence of hair cycling throughout a mammal’s lifetime [28]. Thus, the potential exists to construct HFs from epithelial and mesenchymal stem cells derived from adult tissues [29,30]. In fact, during the formation of HFs, the epidermis first forms placodes along with dermal condensates, precursors of dermal papillae, and permanent mesenchymal units of HFs [31]. Hair germs are then formed from the cells of placodes, and dermal papillae are enclosed by matrix cells, which produce the inner root sheath and outer root sheath that directly contact the basement membrane and are topologically contiguous with the basal layer of the epidermis [15,32]. Our organoids highly expressed Sox2, the earliest marker of incipient dermal condensates, and its descendant, dermal papillae, a crucial component of the HF bulb [1]. Additionally, we detected the presence of putative bulge stem cells (LHX2+, KRT15+) [33,34], a permanent, anatomically distinct region where pluripotent HF stem cells are preserved in a relatively quiescent state [35,36]. These cells give rise to all epithelial lineages of the skin, including keratinocytes and hair [37]. The bulge area is also a reservoir of melanocyte stem cells, which regulates melanocyte repopulation to maintain hair pigmentation [38]. These findings showed that our skin organoids could provide a good recapitulation of HFs. In our skin organoids, a network composed of sensory neurons and Schwann cells was generated, forming nerve-like bundles targeting Merkel cells in HFs. Since the crucial role of Merkel cells in touch sensation has been elucidated [39], this evidence showed the ability of the HF skin organoids to mimic the sensory function of in vivo tissues. Additionally, previous research has reported that signaling from the nervous system promotes HF development through the secretion of mediators [15,40]. The presence of neuron cells in our skin organoids is consistent with previous findings, suggesting that HF growth could be greatly facilitated.
In comparison with a study employing solely floating culture to establish a human hair-bearing skin model using hiPSCs, our combined approach generated HF organoids in a shorter time. To be specific, the previous complete 3D culture system involved a 20-day quiescence period, from day 50 to 70, before the extension of hair-germ-like buds [16]. In our study, early hair placode structures were yielded by day 60. Additionally, the stratification of skin, pigmentation, and development of a sensory neuron network were all comparable. Therefore, by establishing embryoid bodies in floating conditions and then allowing the induction steps to proceed in an adherent environment, the formation and development of hair placodes could be promoted, giving rise to HF organoids with the full potential to mimic native tissues.
Taken together, the 3D HFs in skin organoids generated by this approach could mimic distinct skin layers, along with prominent features of HFs, including pigmentation and touch sensation. By combining floating with adherent culture, the procedure could be simplified, and the culture time could be reduced. This achievement paves the way for further research targeting HF stem cells and optimization of HF maturation, in an attempt to develop treatments for alopecia.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
BSM is supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (2021R1I1A3047154) and by the Ministry of Oceans and Fisheries, Korea (Grant No. 2021-2615). ISK is supported by the National Research Foundation of Korea, which is funded by the Korean government (NRF-2018-R1A6A1A-03024314).
Author contributions
Conceptualization: JMK, ISK, BSM; Data curation: BSM; Formal analysis: MTQN, UB; Funding acquisition: ISK, BSM; Investigation: MTQN, UB, BSM; Methodology: BSM; Project administration: BSM; Resources: JMK, ISK; Supervision: BSM; Validation: JMK, ISK; Visualization: BSM; Writing–original draft: MTQN, UB, HS, JMK, ISK, BSM; Writing–review & editing: BSM.
Data availability
Please contact the corresponding author for data availability.
