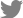Ethical statements: This study was exempted from review by the Institutional Review Board (IRB) of Catholic Medical Center (IRB No: KC18TNSI0033). Written informed consent was obtained from the patients to participate in the study.
Patient information: A 50-year-old man visited The Catholic University of Korea, Seoul St. MaryŌĆÖs Hospital (Seoul, South Korea) on December 11, 2017, for the evaluation of an incidentally found right upper lobe (RUL) pulmonary nodule (
Fig. 1). He was asymptomatic non-smoker and had no notable history of toxicological exposure, and there were no anomalous findings in his family medical history.
Clinical findings: No abnormal findings were seen in the initial physical examinations.
Diagnostic assessment: No distant metastases were found on brain magnetic resonance imaging (MRI) and positron emission tomography/computed tomography scans performed to confirm the patient's lung cancer stage before surgery. The mass was removed by thoracoscopic RUL lobectomy on January 10, 2018. Postoperative pathology confirmed a stage Ib (pT2aN0M0) poorly differentiated carcinoma, according to the 8th American Joint Committee on Cancer staging system. Moreover, lymphatic and vascular invasion were found. An EGFR mutation test was performed on the biopsy sample, and an exon21p.L858R mutation was detected.
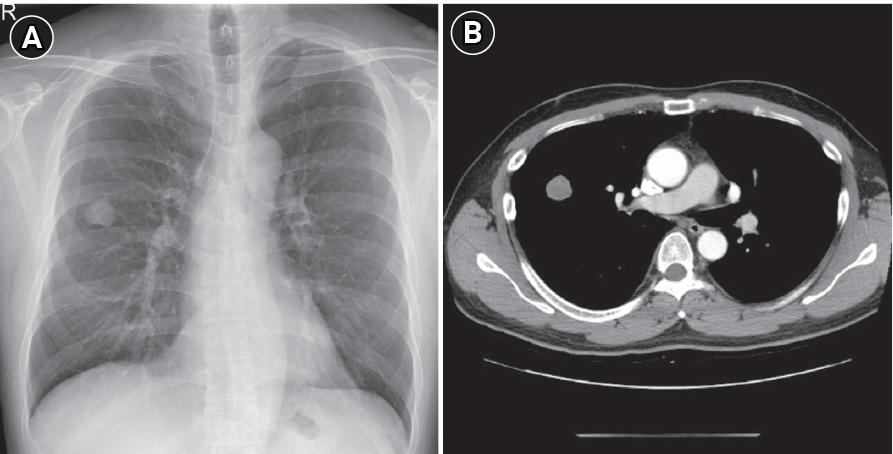
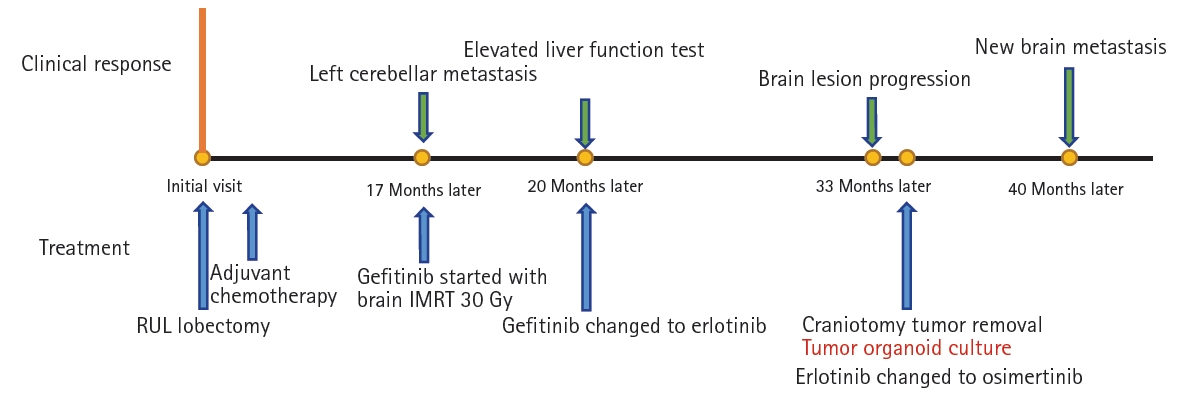
Therapeutic intervention and timeline: This patient received 4 cycles of adjuvant chemotherapy after surgery and was regularly followed up at an outpatient clinic. Brain MRI performed 17 months after surgery showed left cerebellar metastasis (
Fig. 2A). The patient received intensity-modulated radiation therapy with gefitinib based on the previously confirmed
EGFR mutation, and the brain lesion decreased in size after treatment, showing partial response (
Fig. 2B). While taking gefitinib, the patientŌĆÖs aspartate aminotransferase (AST) level rose to 7 times higher than the normal range. The tyrosine kinase inhibitor was changed from gefitinib to erlotinib. Thirty-three months after surgery, growth of the left cerebellar metastasis was noted on a brain MRI examination conducted to evaluate the treatment response (
Fig. 2C). The patient underwent craniotomy with left cerebellar tumor removal in 34 months after lung cancer surgery. Two
EGFR mutations, exon21p.L858R and exon20p.T790M, were detected in the brain metastasis tissue. Erlotinib treatment was switched to osimertinib. Despite the change to osimertinib, a new brain metastasis was revealed on MRI (
Fig. 2D) after 7 months.
Fig. 3 summarizes the patientŌĆÖs clinical course (
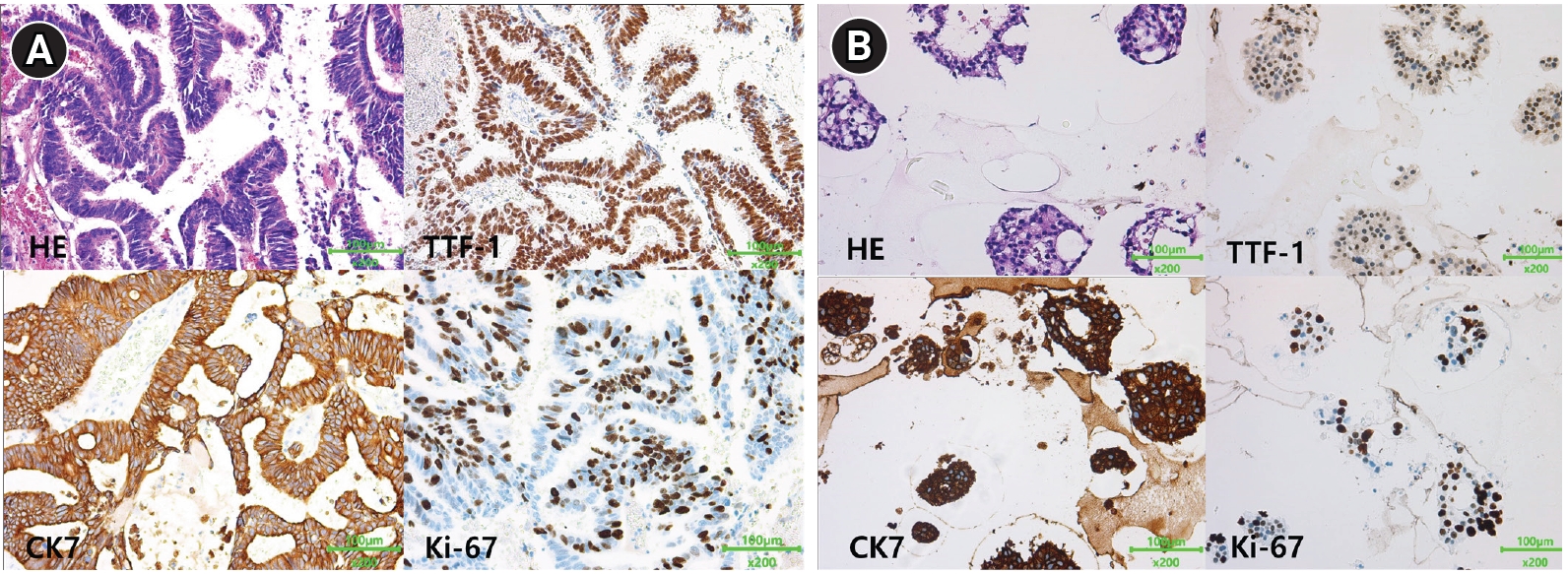
Fig. 3). PDO culture was performed from this resected brain metastatic tissue. The tumor sample was cultured in modified airway organoid medium (Cellvitro; Thermo Fisher, Waltham, MA, USA) for 18 days. Immunohistochemistry staining revealed that the organoid had maintained the morphological and molecular characteristics of the primary tumor tissue. (
Fig. 4). After single-cell dissociation of the cultured organoids from the patient, the presence of the same
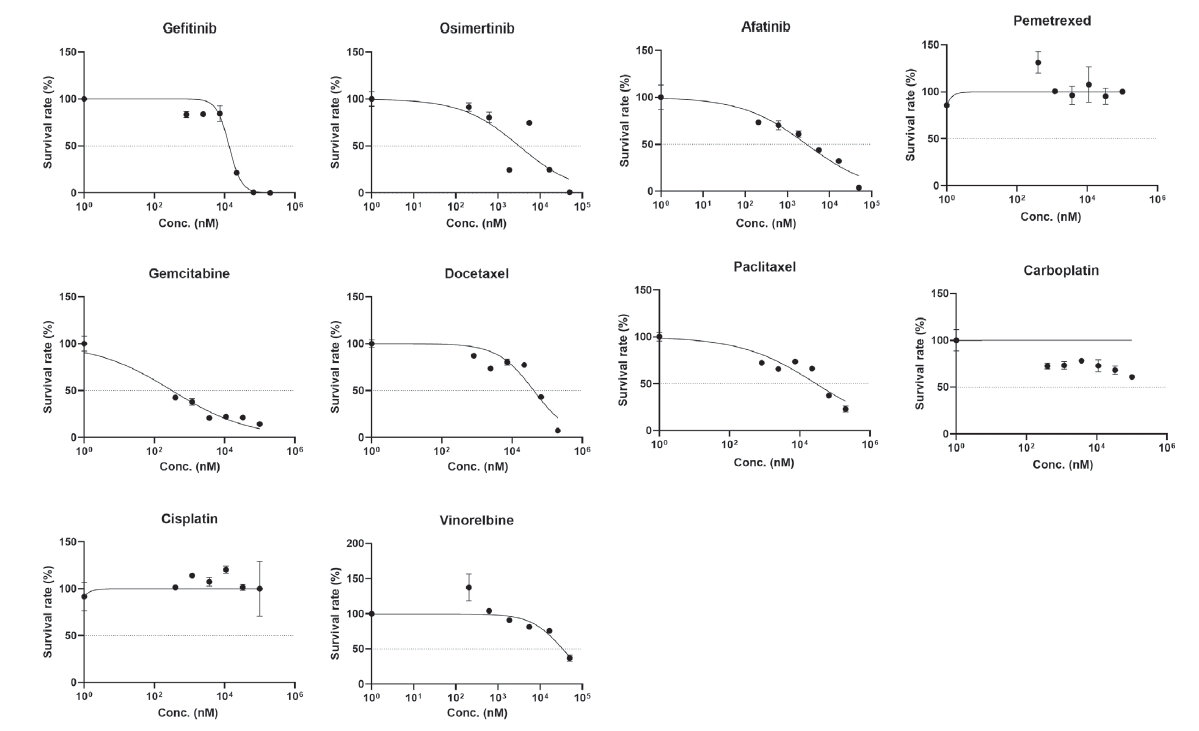
EGFR mutation (p.L858R) as in the parental tissue was confirmed. In order to build tumor organoids within a shorter period of time and to evaluate drug sensitivity promptly, the following method was used. After splitting the patientŌĆÖs primary tumor tissue, dissociation was performed, and 3,000 to 5,000 cells were loaded into a 384-pillar chip for drug screening. Next, cells were cultured in the pillars for 3-5 days and observed with light microscopy. Once it was determined that organoids had formed, anticancer drugs were administered immediately after culture, and susceptibility to multiple anticancer drugs was evaluated by cell viability using calcein fluorescence staining and a cell titer with a three-dimensional assay after 3 days. For 10 anticancer drugs, the survival fraction of tumor cells according to drug concentration was statistically analyzed, and area under the curve values were calculated for each drug (
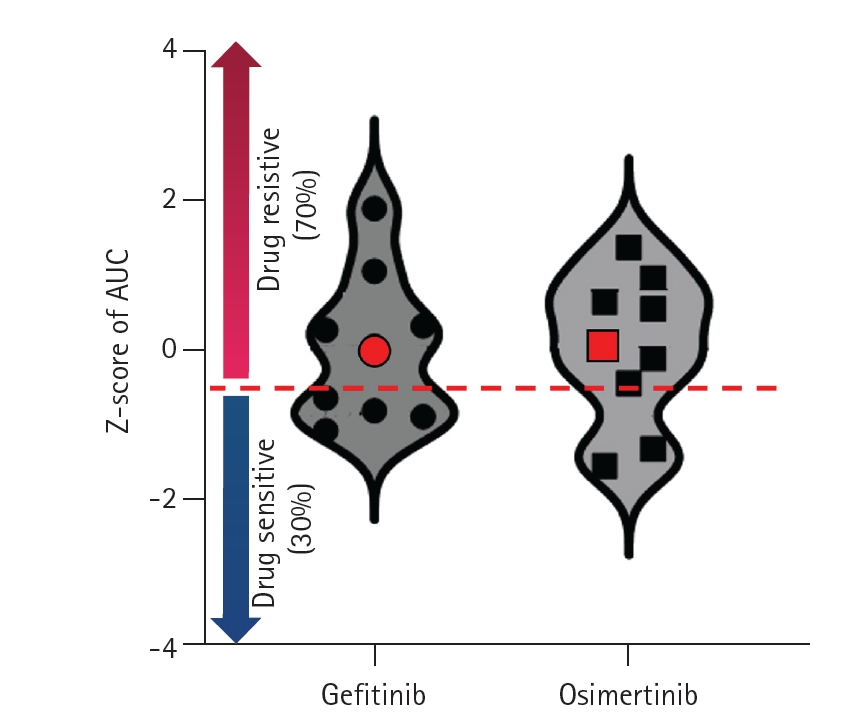
Fig. 5). We prepared a distribution map of drug susceptibility using tumor organoids derived from 9 other previous patients. In the drug sensitivity distribution diagram, this case showed resistance to gefitinib and osimertinib (
Fig. 6).
Follow-up and outcomes: This anticancer sensitivity profile in PDOs was correlated with patientŌĆÖs actual clinical response. He underwent 2 additional operations to remove brain metastases, and the transition to small cell carcinoma was confirmed during the final surgery.















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print