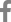|
 |
| Organoid > Volume 3; 2023 > Article |
|
Abstract
Background
Taste buds are a complex organ and require a plethora of growth factors for their development, homeostasis, and regeneration. Taste bud organoids provide a platform for understanding their development, disease and regeneration.
Methods
In this study, we focused on identifying the localization of receptors involved during taste bud development in taste bud organoids, either in an extracellular matrix scaffold (Matrigel) or in the absence of a scaffold with suspension culture.
Results
Compared to Matrigel-cultured organoids, suspension organoids showed stable expression of nerve growth factor receptor (NGFR) cells, which are important for innervation. Transporters for glucose metabolism, such as GLUT1, GLUT2, and the insulin receptor (IGF1R), were observed in suspension-cultured organoids. Furthermore, immunostaining for downstream phosphorylated signaling molecules indicated that the NGFR and IGFR pathways were functional and active in the organoids.
Taste is an important sense for an organismŌĆÖs survival. Taste is detected by taste receptor cells present in the taste buds embedded in the taste papillae, which are replaced continuously [1]. Leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5) is considered to be a marker of stem/progenitor cells in the posterior tongue of mice, and Lgr5-positive cells have been used to generate taste bud organoids bearing functional taste receptor cells [2-4].
Conventional organoid culture methods include extracellular matrix scaffolds, such as Matrigel, to support the three-dimensional growth of organoids. However, in organoids generated using this method, the apicobasal polarity was altered, such that the apical surface of the cells was enclosed inside the organoid. Taste receptor cells, which are functional sensory cells, were encased inside the organoids, which constituted the greatest barrier to assessing organoid functionality. To overcome this problem, Co and colleagues developed the suspension culture technique for enteroids, which enabled the reversal of the apicobasal polarity of the enteroids while maintaining the spheroid structure [5]. Applying this technique to taste bud organoids resulted in a similar phenomenon, leading to the reversal of the apicobasal polarity of taste bud organoids and functional accessibility to tastants compared to Matrigel-cultured taste bud organoids [6].
Numerous factors collectively participate in the development of mouse taste buds. Neurotrophins control neurons that innervate the taste buds [7]. Neurotrophins bind to various receptors, including the nerve growth factor receptor (NGFR) p75, which is considered a pan-neurotrophin receptor. Previous studies have shown that p75 promotes tongue innervation and regulates axon branching [8]. Furthermore, research has also shown that mice lacking the p75 receptor do not develop the full complement of taste buds [9]. Hence, we investigated whether taste receptor cells in organoids expressed these receptors, which could aid in nerve innervation upon transplantation of the organoids.
Previous studies have reported that insulin-like growth factor receptor 1 (IGF1R) is expressed in taste buds. Insulin-like growth factors (IGF1 and IGF2), which are produced by ganglia of the innervating nerves, bind to IGF1R and influence the proliferation of keratinocytes and regulate the number of papillae or taste buds during development [10,11]. Glucose is a major biomolecule that is absorbed by glucose transporters present on the cell membrane. Previous studies have shown that taste receptor cells in the circumvallate papillae of rodents express glucose transporters, such as GLUT1 and GLUT2 [12,13]. Hence, we investigated the presence of these transporters in taste bud organoids, which could serve as a useful platform for assessing biomolecule transport, uptake, and metabolism. Since taste bud organoids could serve as a convenient platform for taste research, the present study investigated the presence of the factors involved in taste bud development in Matrigel- and suspension-cultured organoids, aiming to identify an efficient model to study taste bud development.
Ethics statement: No experiments involving human subjects were performed during this study. All experiments were performed according to the guidelines of the Intramural Animal Use and Care Committee of the College of Dentistry, Yonsei University (2019-0312).
Adult mice were housed in a temperature-controlled room (22┬░C) under artificial illumination on a regular 12-hour day/night cycle and 55% relative humidity with access to food and water ad libitum. The mice used in the study were adult (6-8 weeks┬▒5 days) male and female animals.
Tongues from sacrificed adult mice were dissected and injected withŌĆē~0.5 mL of dispase II (2.2 unit/mL; Roche, Basel, Switzerland) in phosphate-buffered saline (PBS) for 25 minutes at 37┬░C. The tongue epithelium was detached gently from the underlying tongue mesenchyme. The epithelium of circumvallate and foliate papillae was dissected and incubated with TrypLE Express for 30 minutes at 37┬░C and centrifuged at 800 rpm for 20 minutes. The cell pellet was resuspended in Matrigel and seeded onto 24-well culture plates (50 ╬╝L of Matrigel). Matrigel was allowed to polymerize for at least 10 minutes at 37┬░C. A taste culture medium based on DMEM/F12 supplemented with N2 (1%), B27 (2% [vol/vol]), R-spondin-1 (200 ng/mL), noggin (100 ng/mL), jagged-1 (1 ╬╝M), Y27632 (10 ╬╝M), N-acetylcysteine (1 mM), and epidermal growth factor (50 ng/mL) was added to the plate. The growth medium was changed every 3 days. For passage culture, organoids were transferred into TrypLE solution and dissociated into small pieces mechanically using a fine glass pipette. The solution was then passed through a cell strainer to obtain single cells. Cells were pelleted by centrifuging at 600 ├Ś g for 5 minutes. Single cells were re-embedded into fresh Matrigel and plated in 24-well plates.
Single lingual epithelial cells were embedded and cultured in Matrigel for 4 days with growth medium, as mentioned above. The Matrigel-embedded organoids were washed with ice-cold basal medium to break down the Matrigel by inverting and subsequently incubating on ice for 20 minutes. The organoids were gently centrifuged at 900 rpm for 5 minutes at 4┬░C, and the supernatant was removed carefully using a Pasteur pipette without disturbing the pelleted organoids. Fresh ice-cold basal medium was added for washing. This process was repeated three times. The organoids were resuspended in growth medium and transferred to ultra-low attachment 96-well culture plates (Costar; Corning Inc., Corning, NY, USA). Suspended organoids were cultured at 37┬░C with 5% CO2.
The organoids were fixed in 4% paraformaldehyde and processed until Optimal Cutting Temperature (OCT) compound using standard procedures. Sections (7 ╬╝m) were prepared for immunostaining. For immunostaining, the slides were boiled in citrate buffer (pH 6.0). Blocking was carried out using 1% goat serum or 5% bovine serum albumin in PBS. The slides were incubated with antibodies against GLUT1 (1:200), GLUT2 (1:200), IGF1R (1:200), NGFR (1:200), K8 (1:200), Ecad (1:200), pMAPK (1:100), and pMEK (1:100) at 4┬░C overnight. The following day slides were washed and sequentially incubated with a secondary antibody (1:200; Invitrogen, Waltham, MA, USA) and counterstained with DAPI to visualize nuclei. The sections were examined using a confocal laser microscope (Leica DMi8).
The total RNA was extracted using the TRIzol reagent (#15596-026; Thermo Fisher Scientific, Waltham, MA, USA). The extracts were reverse-transcribed using Maxime RT PreMix (#25081; iNtRON, Seongnam, Korea). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using a StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The expression levels of each gene are expressed as normalized ratios against the B2m housekeeping gene.
To alter their apicobasal polarity, the organoids cultured in Matrigel were transferred to suspension culture in a low-attachment dish after 96 hours (Fig. 1A). To examine the altered morphology between the two culture methods, hematoxylin and eosin staining was performed. As reported in our previous study [6], a keratinized inner core was observed in the Matrigel-cultured organoids that was not seen in the suspension-cultured organoids (Fig. 1B and 1C). Furthermore, taste receptor cells expressing keratin 8 (K8) were localized inside the Matrigel-cultured organoids, but on the periphery in the suspension-cultured organoids, indicating alteration of apicobasal polarity after suspension culture (Fig. 1D and 1E). The NGFR p75 is a pan-neurotrophin receptor that binds to neurotrophins to promote neuronal development, survival, and differentiation [14]. In the Matrigel-cultured taste bud organoids, NGFR p75 expression was observed on the periphery of the organoids, and NGFR p75-positive cells were not observed inside the Matrigel organoids where mature taste receptor cells were localized (Fig. 1F). In contrast, localization of NGFR p75 was observed on the periphery of the taste bud organoids and inside the suspension-cultured organoids (Fig. 1G). These results indicate that the NGFR p75 was co-localized with K8-positive taste receptor cells in the suspension-cultured taste bud organoids.
Glucose transporter isoforms such as GLUT1 and GLUT2 are expressed by taste receptor cells [12,13]. To investigate whether taste bud organoids also expressed glucose transporters, immunohistochemistry was performed for GLUT1 and GLUT2 transporter channels with the epithelial cell marker E-cadherin. GLUT1 was observed to co-localize with epithelial cells both inside and on the periphery of Matrigel- and suspension-cultured organoids (Fig. 2A and 2D). GLUT2 staining co-localized with E-cadherin-expressing epithelial cells on the periphery of Matrigel-cultured organoids, whereas the suspension-cultured organoids showed co-localization both on the periphery and inside the organoids (Fig. 2B and 2E). NGFR p75 co-localized with E-cadherin-positive epithelial cells on the periphery of Matrigel-cultured organoids (Fig. 2C). In contrast, after suspension culture of taste bud organoids, NGFR p75 was observed both on the periphery and inside the organoids (Fig. 2F). IGF1R is expressed in the taste buds and interacts with insulin-like growth factors, which are secreted by the neural ganglia and are required for cell growth and differentiation [11]. Immunohistochemical staining revealed that IGF1R was not detected in the Matrigel-cultured taste bud organoids (Supplementary Fig. 1A). However, IGF1R was partially observed in the cells of the suspension-cultured organoids (Supplementary Fig. 1B). These results indicate that the taste epithelial cells in the suspension-cultured organoids expressed GLUT1 and GLUT2 like in vivo taste buds.
A subtype of type II taste receptor cells, belonging to the type I taste G protein-coupled receptor family, forms a dimer that functions as a sweet, bitter, or umami receptor. Sweet taste is mediated by the heteromeric complex of Tas1r2 (taste receptor type 1, member 2) and Tas1r3 (taste receptor type 1, member 3) [15,16]. After 14 days of culture, the expression level of sweet taste receptor genes was significantly higher in suspension-cultured organoids than in Matrigel-embedded organoids (Fig. 3A). Furthermore, to validate the immunohistochemistry data, which showed increased expression of growth factor receptors and glucose transporters after suspension culture, the expression levels of Ngfr and Glut2 were analyzed. Both Ngfr and Glut2 were upregulated in suspension-cultured organoids compared to Matrigel-embedded organoids (Fig. 3B). Thus, taste receptor cells, growth factor receptors, and glucose transporters were maintained and enhanced after suspension culture.
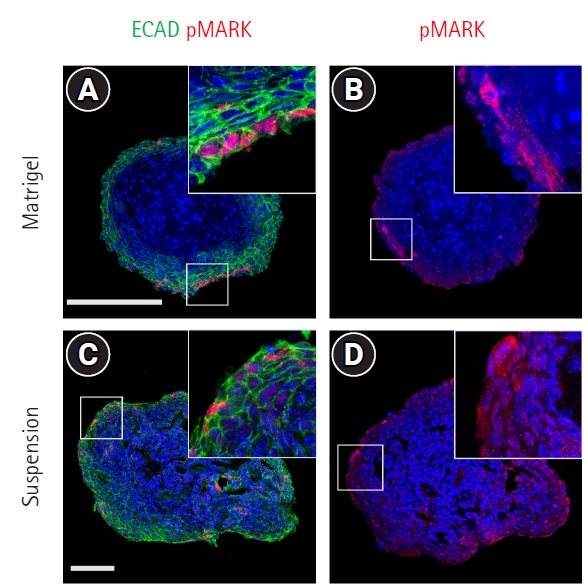
Previous studies have shown that NGFR and IGF1R signaling occurs via distinct receptor tyrosine kinases, which then activate common intracellular signaling pathways, such as Ras, mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase, and the serine-threonine kinase Akt [17]. To examine the activation state of these receptors in the taste bud organoids, immunostaining for intermediate signaling molecules was performed using phospho-specific antibodies. Immunostaining showed localization of phospho-MAPK and phospho-MEK in the nucleus of a subset of peripheral cells in the Matrigel-embedded organoids (Fig. 4A and 4B). In the suspension-cultured organoids, phospho-MAPK and phospho-MEK were observed in peripheral cells, as well as in the cells present inside the organoids (Fig. 4C and 4D). These data indicate that NGFR p75 and IGF1R are functionally active in taste bud organoids.
The purpose of this study was to confirm that suspension-cultured taste bud organoids exhibited both improved morphology and improved growth factor localization. To investigate the localization of various growth factors, taste bud organoids were generated by the conventional method using Matrigel as an extracellular matrix scaffold or by culturing the organoids in suspension without Matrigel.
Suspension culture reverses the apicobasal polarity of organoids, facilitating access to the apical surface while maintaining the spheroid structure, unlike conventional Matrigel-cultured organoids. Recent studies have reported that suspension-cultured organoids induced efficient organoid growth, thereby increasing the yield and reducing costs while maintaining genotypic and phenotypic stability [18,19].
Neurotrophins play a vital role during taste bud development by guiding gustatory axons towards specific regions of the tongue, such as taste placodes, and by controlling the number of neurons innervating the taste buds [7,20]. NGFR p75, which is considered a pan-neurotrophin receptor, can bind to brain-derived neurotrophic factor and neurotrophin-4. NGFR p75 regulates the branching of axons and innervation patterns in the tongue during taste system development [8]. Hence, the localization of NGFR p75 was investigated in Matrigel- and suspension-cultured taste bud organoids. In the Matrigel-cultured organoids, NGFR p75 was observed in the peripheral cells (Fig. 1E), where no K8-positive taste receptor cells were localized. However, NGFR p75 was observed both on the periphery and inside the suspension-cultured organoids, indicating the capability of innervation upon transplantation, as observed in our previous paper (Fig. 1F) [6].
GLUTs play a major role as metabolic sensors in various organs such as the gut, brain, and pancreas [21,22]. Previous studies have shown the expression of GLUT1 and GLUT2 in rodent taste receptor cells, suggesting that taste receptor cells might participate in glucose sensing and glucose homeostasis [12,13,23]. To confirm the localization of GLUT1 and GLUT2, immunohistochemistry was performed in both Matrigel- and suspension-cultured taste bud organoids. GLUT1 localization was similar in suspension-cultured taste bud organoids and Matrigel organoids (Fig. 2A and 2D). GLUT2 was not observed inside the Matrigel-cultured organoids, which is the putative region of matured taste receptor cells (Fig. 2B), whereas GLUT2 was observed both inside the suspension-cultured taste bud organoids and along their periphery, unlike the Matrigel-cultured organoids (Fig. 2E). Previous studies have reported that IGF1R is highly expressed in taste buds, and its ligands IGF1 and IGF2 are present in the surrounding nerve fibers [11,24]. Interestingly IGF1R-positive cells were partially observed inside the suspension-cultured taste bud organoids, but not in the Matrigel-cultured organoid (Supplementary Fig. 1). We observed active downstream pathways of IGF1R in the Matrigel-embedded organoids, but that may have been due to the presence of shared downstream targets, such as the NGFR pathway, which was active in both organoid systems.
In summary, this study showed that suspension-cultured taste bud organoids have significant advantages over Matrigel-cultured organoids, in terms of both morphology and the expression of growth factors, for maintaining themselves after transplantation in vivo.
Supplementary Information
Supplementary materials are presented online (available at https://doi.org/10.51335/organoid.2023.3.e9).
Supplementary Fig. 1.
Insulin growth factor receptor (IGF1R) is expressed in suspension-cultured organoids. (A, B) 14-day cultured taste bud organoids are immunostained by anti-IGF1R. (A) IGF1R is not expressed in the cells of Matrigel-cultured organoid. (B) IGF1R expressing cells are observed in suspension-cultured taste bud organoid, n=3, scale bar:100 ┬Ąm.
NOTES
Funding
A National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2022R1A2B5B03001627) supported this work.
Fig.┬Ā1.
Apicobasal polarity alteration and localization of nerve growth factor receptor (NGFR) p75 in organoids. (A) Schematic diagram describing the culture of Matrigel-embedded and suspension-cultured organoids from the mouse circumvallate papilla (CVP). (B, C) Hematoxylin and eosin staining of Matrigel-embedded and suspension-cultured organoids after 14 days of culture. A keratinized inner core is observed in the Matrigel organoids, whereas the suspension-cultured organoids lack a keratinized inner core. (D, E) Immunohistochemical staining of keratin 8 (K8), a pan-taste receptor cell marker, shows that taste receptor cells are localized on the apical surface in suspension-cultured taste bud organoids, unlike the Matrigel organoids. (F, G) NGFR p75 staining is observed in the periphery of the Matrigel organoids, whereas it is observed both in peripheral cells and in the cells inside the suspension-cultured organoids. n=3, scale bar: 100 ┬Ąm.
Fig.┬Ā2.
Suspension-cultured taste bud organoids express glucose transporters and nerve growth factor receptor. (A-F) Fourteen-day cultured taste bud organoids are immunostained by anti-GLUT1, GLUT2, nerve growth factor receptor (NGFR) p75, and E-cadherin. (A) GLUT1 expression is observed in peripheral epithelial cells and a few cells inside the Matrigel-embedded organoids. (D) GLUT1 is observed in the epithelial cells on the periphery, as well as the inner epithelial cells of suspension-cultured organoids, where taste receptors and progenitor cells are localized. (B) GLUT2 expression is observed on the basal side of peripheral cells in Matrigel-embedded organoids. (E) GLUT2 is detected on the periphery and in the cells inside the suspension-cultured taste bud organoids. (C, F) NGFR p75 is expressed in most of the peripheral epithelial cells in the Matrigel-embedded organoids, and both in the peripheral cells and some of the inner cells in the suspension-cultured organoids. n=3, scale bar: 100 ┬Ąm.
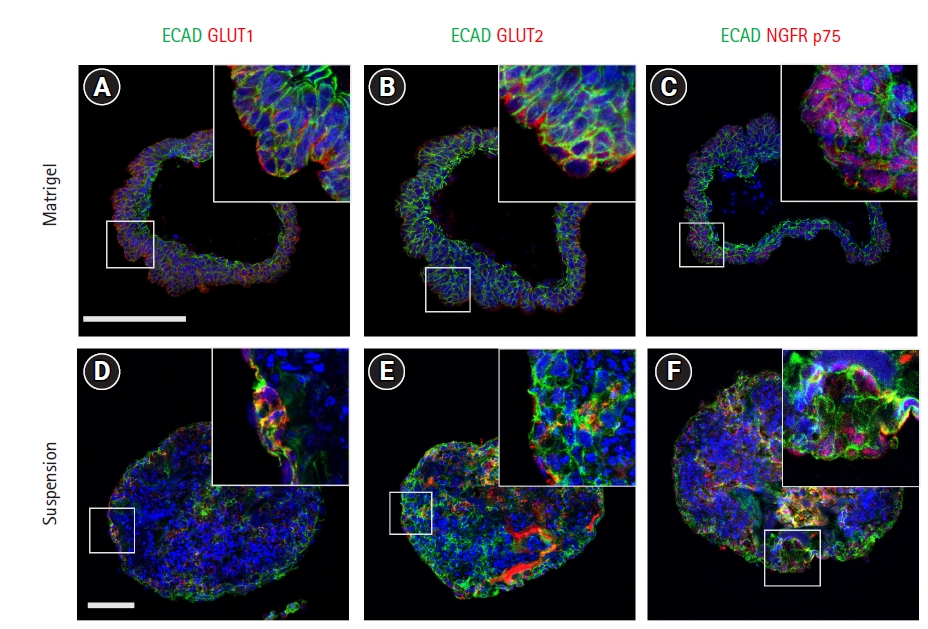
Fig.┬Ā3.
Suspension-cultured organoids show higher expression of receptors and transporters. (A) The expression of receptors for subtypes of type II taste receptor cells, Tas1r2 and Tas1r3, is significantly higher in suspension-cultured organoids than in Matrigel-embedded organoids. (B) The growth factor receptor Ngfr and glucose transporter Glut2 also show higher expression levels in the suspension-cultured organoids than in the Matrigel-embedded organoids. Data are represented as mean┬▒standard deviation; real-time quantitative polymerase chain reaction data were compared using the unpaired two-tailed t-test. *p<0.01, **p<0.001, ***p<0.0001.
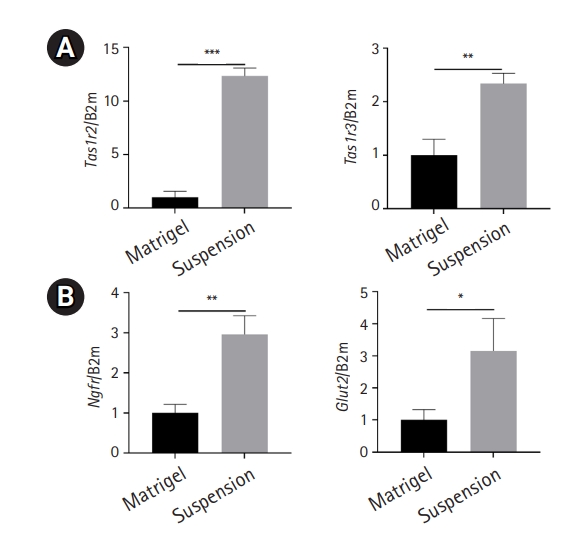
Fig.┬Ā4.
Nerve growth factor receptor (NGFR) p75 and insulin growth factor receptor (IGF1R) receptors are functionally active in the taste bud organoids. (A-D) Fourteen-day cultured taste bud organoids are immunostained by anti-pMAPK1, pMEK and E-cadherin. (A, B) Localization of pMAPK and the MEK-downstream phosphorylated intermediate of the NGFR p75 and IGF1R pathways in the nucleus of peripheral cells in the Matrigel-embedded organoids and (C, D) suspension-cultured organoids. n=3, scale bar: 100 ┬Ąm.
References
1. Barlow LA. Progress and renewal in gustation: new insights into taste bud development. Development 2015;142:3620-9.




2. Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells 2013;31:992-1000.




3. Takeda N, Jain R, Li D, Li L, Lu MM, Epstein JA. Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One 2013;8:e66314.



4. Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc Natl Acad Sci U S A 2014;111:16401-6.



5. Co JY, Margalef-Catal├Ā M, Li X, Mah AT, Kuo CJ, Monack DM, et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep 2019;26:2509-20.



6. Adpaikar AA, Zhang S, Kim HY, Kim KW, Moon SJ, Lee JM, et al. Fine-tuning of epithelial taste bud organoid to promote functional recapitulation of taste reactivity. Cell Mol Life Sci 2022;79:211.




7. Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol 1998;398:13-24.


8. Fei D, Huang T, Krimm RF. The neurotrophin receptor p75 regulates gustatory axon branching and promotes innervation of the tongue during development. Neural Dev 2014;9:15.




9. Krimm RF. Mice lacking the p75 receptor fail to acquire a normal complement of taste buds and geniculate ganglion neurons by adulthood. Anat Rec A Discov Mol Cell Evol Biol 2006;288:1294-302.



10. Stachelscheid H, Ibrahim H, Koch L, Schmitz A, Tscharntke M, Wunderlich FT, et al. Epidermal insulin/IGF-1 signalling control interfollicular morphogenesis and proliferative potential through Rac activation. EMBO J 2008;27:2091-101.



11. Biggs BT, Tang T, Krimm RF. Insulin-like growth factors are expressed in the taste system, but do not maintain adult taste buds. PLoS One 2016;11:e0148315.



12. Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat 2011;219:243-52.



13. Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res 2011;345:243-52.



14. Kawakoshi K, Suzuki Y, Okumura K, Shibata T, Takeda M. Expression of nerve growth factor and neurturin, and their receptors in mouse taste buds. J Oral Biosci 2005;47:157-67.

15. Belloir C, Brul├® M, Tornier L, Neiers F, Briand L. Biophysical and functional characterization of the human TAS1R2 sweet taste receptor overexpressed in a HEK293S inducible cell line. Sci Rep 2021;11:22238.




16. Ahmad R, Dalziel JE. G protein-coupled receptors in taste physiology and pharmacology. Front Pharmacol 2020;11:587664.



17. Jones DM, Tucker BA, Rahimtula M, Mearow KM. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem 2003;86:1116-28.


18. Price S, Bhosle S, Gon├¦alves E, Li X, McClurg DP, Barthorpe S, et al. A suspension technique for efficient large-scale cancer organoid culturing and perturbation screens. Sci Rep 2022;12:5571.




19. Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, et al. Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development 2019;146:dev172361.




20. Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139-58.



21. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 2007;8:113-28.



22. Scheepers A, Joost HG, Sch├╝rmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr 2004;28:364-71.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,753 View
- 58 Download
- ORCID iDs
-
Anish Ashok Adpaikar

https://orcid.org/0000-0001-6018-0427Han-Sung Jung

https://orcid.org/0000-0003-2795-531X - Related articles
-
Microengineered organoids: reconstituting organ-level functions in vitro2023 ;3()




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement
Supplement Print
Print