Kidney organoids: development and applications
Article information
Abstract
Since the first publication on generating kidney-like cell aggregates from pluripotent stem cells, various modifications have been made to develop more complex and detailed kidney structures. In contrast to earlier models that featured nephron-like structures, these advances have improved the differentiation efficiency and similarity to the human kidney. Presently, kidney organoids contain not only nephrons and ureteric buds but also stromal cells. These organoids mimic the structural similarities and developmental processes of the kidneys, while reflecting their physiological properties. Kidney tubuloids derived from adult stem cells offer the advantage of long-term culture and expansion, but they include only tubular structures and lack glomerular components. In this review, we discuss the induction protocols for kidney organoids and tubuloids, as well as their potential applications in understanding kidney development, renal pathogenesis, and drug screening.
Introduction
The kidney is a complex organ that becomes highly organized during development. Renal complexity is characterized by several functionally and structurally distinct segments. One functional component of the kidney, the nephron, carries out tasks such as filtration and reabsorption. The human kidney contains approximately one million nephrons, each composed of at least 20 different cell types. These include the major components of the glomerulus, proximal and distal tubules, loop of Henle, and collecting duct [1].
The embryonic kidney consists of the metanephric mesenchyme and ureteric buds. During the metanephric development process, epiblasts expressing T (brachyury) give rise to nascent mesoderm on embryonic day (E) 7.5. The metanephros originates from the intermediate mesoderm, which expresses odd-skipped related transcription factor 1 (Osr1). This intermediate mesoderm then divides into the posterior nascent mesoderm (E8.5), arising from the posterior intermediate mesoderm, and the anterior intermediate mesoderm (E8.5), which gives rise to the ureteric bud generated from the Wolffian duct progenitor (E9.5) [2,3]. The anterior intermediate mesoderm, which expresses LIM homeobox 1 (Lhx1) and paired box gene 2 (Pax2), develops into the ureteric bud lineage, which forms the ureteric epithelium through branching and growth. The posterior intermediate mesoderm, expressing Wilms tumor 1 (Wt1) and homeobox-leucine zipper (Hox11), develops into the metanephric mesenchyme; this gives rise to the cap mesenchyme and interstitial stroma populations (Fig. 1) [4–6].
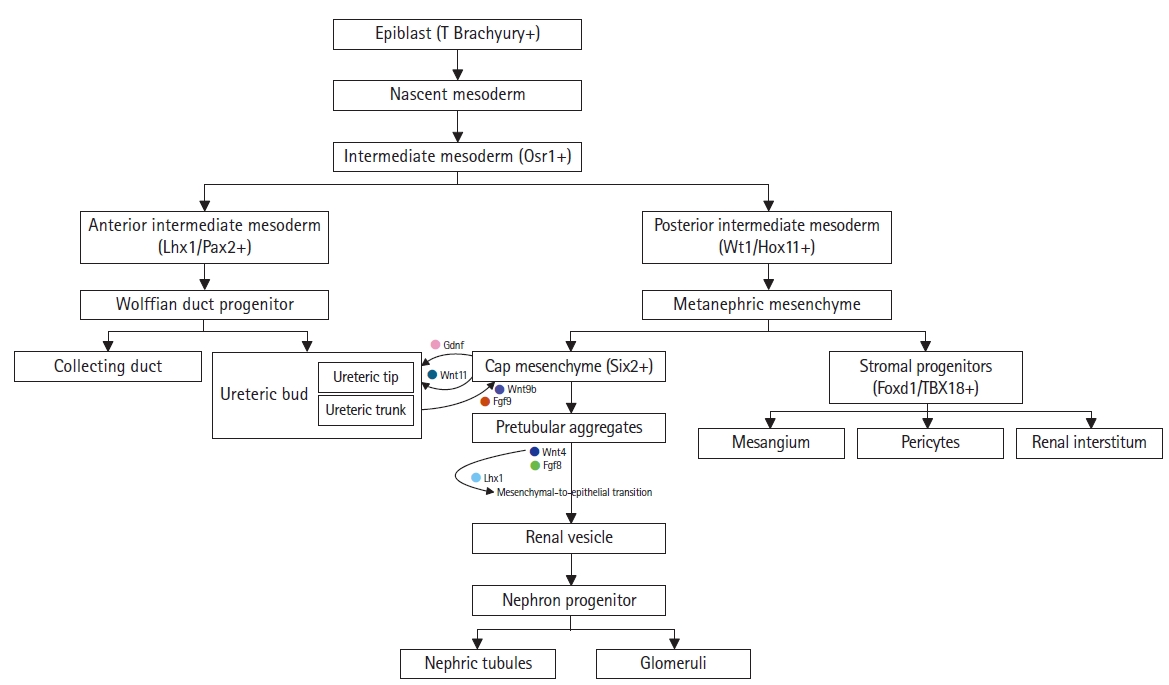
Renal lineage in nephric development. The interaction between the metanephric mesenchyme and the nephric duct is crucial for nephrogenesis. Each compartment sequentially organizes the developmental stages of the ureteric bud or nephron progenitor population. Arrows and key markers represent the signal regulation between each compartment.
On day E10.5 of murine development, the metanephric mesenchyme secretes glial cell line-derived neurotrophic factor (GDNF). This factor promotes the growth and branching of the ureteric bud.
Osr1-expressing cell clusters spatially regulate Six2 expression, which in turn induces the cap mesenchymal population. The SIX homeobox 2 (Six2)-positive cap mesenchyme is formed by the surrounding metanephric mesenchyme adjacent to the tips of the branching ureteric bud. These epithelialize, leading to the sequential formation of pretubular aggregates, renal vesicles, and comma- and S-shaped bodies [2,7]. The cap mesenchyme secretes Wingless family member 11 (Wnt-11), which promotes ureteric bud differentiation. Meanwhile, the ureteric bud secretes Wnt-9b and fibroblast growth factor 9 (FGF9), which differentiate the metanephric mesenchyme into pretubular aggregates. As a result of FGF8 and Wnt4 secretion, Lhx1 activation initiates the mesenchymal-to-epithelial transition, causing the pretubular aggregates to form renal vesicles. These renal vesicles connect the collecting duct and ureter, developing into metanephric nephron progenitors (Fig. 1) [8,9].
The interstitial stroma, another subcompartment of the metanephric nephron, is characterized by the expression of the transcription factor forkhead box D1 (Foxd1). Foxd1- and T-box 18 (TBX18)-positive cell populations give rise to various cell types, including renin cells, vascular smooth muscle cells, pericytes, and mesangial cells (Fig. 1). The stromal population is associated with non-glomerular and glomerular vasculature and promotes ureteric bud development [10–13]. Cross-talk between the metanephric mesenchyme and the ureteric bud is essential for higher-order kidney development [14–16].
Animal models have been developed to improve our understanding of kidney development; however, they present challenges due to interspecies differences from human kidneys, ethical concerns, experimental duration, and cost. Human primary or immortalized cell lines have been employed to investigate the characteristics of specific cell types. However, these cell lines tend to lose their primary properties during culture, resulting in a divergence from their in vivo counterparts. Moreover, observing cell-cell interactions can be difficult. Consequently, a need exists for an enhanced kidney-mimicking model to address the limitations of existing models, and kidney organoids currently fulfill this role.
Kidney organoids include nephron progenitor cells, higher-order organoids, and tubular epithelial organoids. These organoids are derived from pluripotent stem cells (PSCs), including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs). They form self-organizing 3-dimensional (3D) structures that mimic the intricate structures and functions of the kidney [17,18]. The utility of kidney organoids relies on the extent to which the renal cellular identity and complexity of these in vitro models resemble those of the human kidney. The methods for developing kidney organoids have seen substantial advancements in recent years. In this review, we explore the characteristics of these methods and discuss their remaining limitations. Additionally, we address the application of kidney organoids in disease modeling and drug screening (Fig. 2).
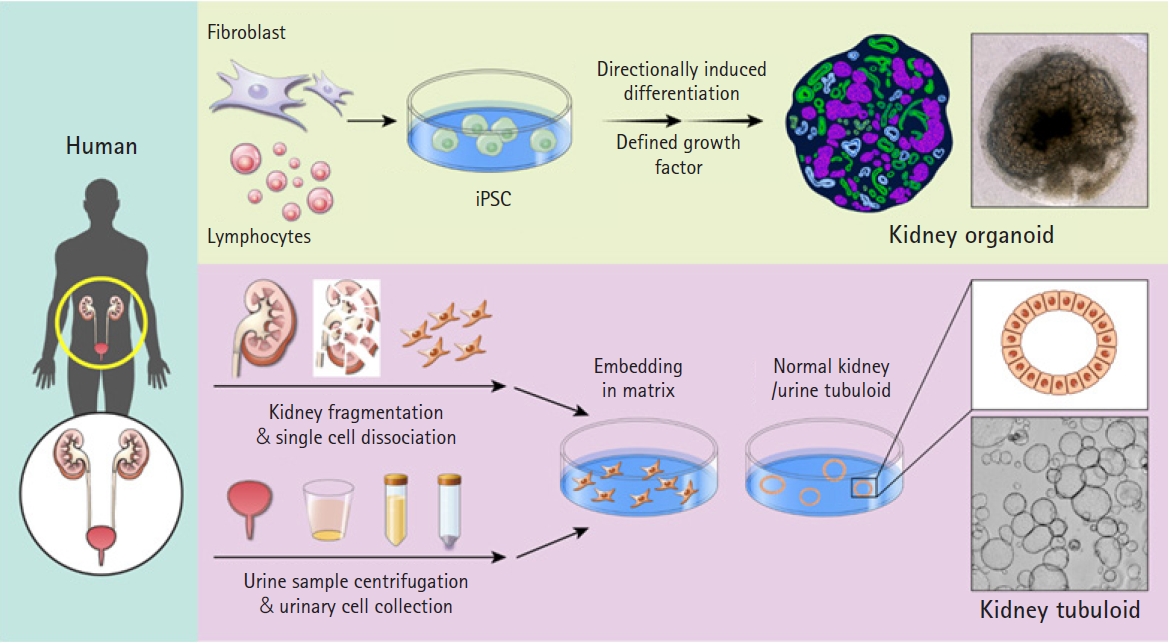
Differentiation of induced pluripotent stem cell-derived kidney organoids and establishment of adult stem cell-derived kidney tubuloids. A comparison of human induced pluripotent stem cell (iPSC)-derived kidney organoids and adult stem cell (ASC)-derived tubuloids is presented. Kidney organoids are generated through directional differentiation in reprogrammed iPSCs derived from human lymphocytes or fibroblasts (top). The kidney tubuloid protocol, established using ASCs, necessitates the dissociation of renal tissue or urine samples. After processing, tubular fragments or urine-derived cells are directly seeded into the matrix and given growth factor treatments to promote the differentiation of only tubular structures (bottom).
Nephron progenitor organoid induction
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Before the development of multicellular kidney organoids, numerous researchers proposed studies to generate a renal single-cell population using PSCs. Kidney cell populations induced from human iPSCs or ESCs were identified, with each cell type including the posterior primitive streak, nephrogenic intermediate mesoderm, and mesenchymal mesoderm. Induced proximal tubular cells and nephron progenitor cell populations were observed to express glomerular and proximal tubular epithelial markers, including claudin, aquaporin, and gamma-glutamyltransferase. Additionally, podocytes generated from iPSCs exhibited protein and messenger RNA expression of podocyte-specific markers. Another study reported that ureteric bud progenitor-like cells generated from iPSCs and ESCs expressed Hoxb7, receptor tyrosine kinase (RET), and GDNF receptor alpha 1 (Gfra1) [19–24]. Meanwhile, the generated primitive “renal organoid,” in which dissociated single cells were aggregated in the embryonic kidney following implantation into the renal capsule of rats with vascular endothelial growth factor treatment, partially displayed kidney characteristics [25].
CHIR99021 (CHIR), a Wnt agonist, is the most commonly used chemical for inducing nephron precursors. CHIR treatment regulates the initial induction into the nascent mesoderm and enables directional induction [26–28]. A high concentration of CHIR stimulates the posterior nascent mesoderm to develop into the posterior intermediate mesoderm, while insufficient activation of CHIR results in the induction of the anterior intermediate mesoderm. The Takasato protocol established the generation of nephron-like structures using a high CHIR concentration to induce metanephric mesenchyme populations (Fig. 3). These nephron organoids expressed renal markers, including WT1+ nephrin (NPHS1)+ podocytes in the glomeruli, cadherin 1 (CDH1)+ uromodulin (UMOD)+ in the loop of Henle, and Lotus tetragonolobus lectin (LTL)+ in the proximal tubules. Additionally, a CDH1+GATA3+ paired box 2 (PAX2)+ collecting duct network was induced, which was surrounded by platelet-derived growth factor receptor alpha (PDGFRA)+ renal early mesangial and CD31+ endothelial cells. The authors also conducted functional assays, including dextran uptake and cisplatin nephrotoxicity assessments, to confirm the maturation of proximal tubules in the nephrons within these organoids [27].
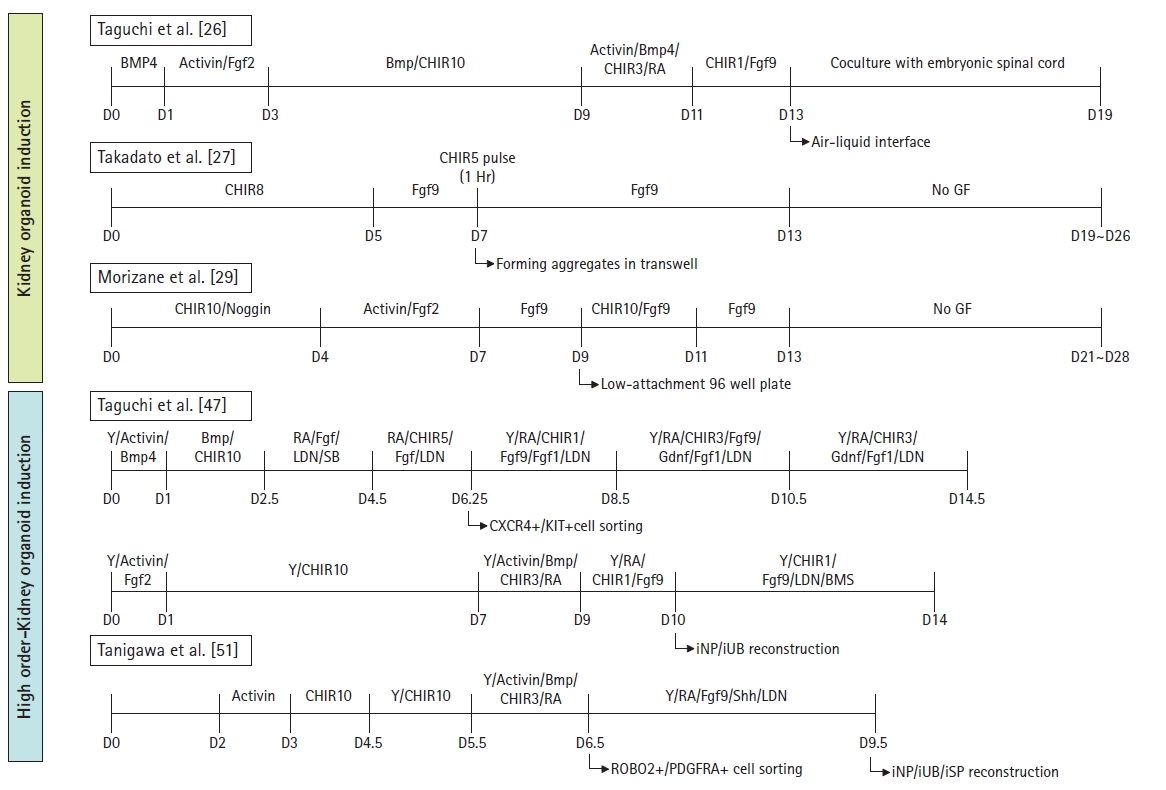
Comparison of human kidney organoid differentiation protocols. This diagram summarizes the protocols used for kidney organoid differentiation, including the higher-order methodology. Each stage of the differentiation protocols is depicted, along with the inducing factors, concentrations, and treatment durations. For each protocol presented, the authors and publication details are provided in a box. BMP4, bone morphogenetic protein 4; Fgf 2,9, fibroblast growth factor 2,9; CHIR, CHIR 99021, Wnt agonist; Hr, hour; GF, growth factor; Y, Y27632, a selective p160 ROCK inhibitor; RA, retinoic acid; LDN, LDN193189, a BMP inhibitor; Gdnf, glial cell-derived neurotrophic factor; CXCR4, C0X0C chemokine receptor type 4; KIT, tyrosine-protein kinase; iNP, induced nephron progenitor; iUB, induced ureteric bud; BMS, BMS493, RA receptor agonist; Shh, Sonic Hedgehog; ROBO2, roundabout guidance receptor 2; PDGFRA, platelet-derived growth factor receptor alpha; iSP, induced stromal progenitor.
Morizane et al. [29] proposed a protocol for inducing the differentiation of nephron-like structures through highly efficient expression of the nephron progenitor cell marker, SIX2. In a 3D culture system using ultra-low attachment 96-well round-bottom plates, nephron progenitor cell populations were induced with 75% to 92% efficiency. Ten to 14 days after the initial point, the nephron progenitor cell population expressed SIX2, and upon FGF9 treatment, these cells differentiated into PAX8+LHX1+ renal vesicle cells (Fig. 3). The induction efficiency for differentiation of the intermediate mesoderm into nephron progenitor cell populations was higher than that reported in previous studies. On day 21 after differentiation, renal vesicle-like clusters formed nephron-like structures with LTL+CDH2+ proximal tubules, NPHS1+ podocalyxin (PODXL)+ podocytes, CDH1+UMOD+ loops of Henle, and CDH1+ POU-homeodomain transcription factor Pou3f3+ Barren BRN1+ distal tubules. Transmission electron microscopy analyses of these organoids revealed ultrastructures including the primary and secondary foot processes of podocytes, brush border-like structures of proximal tubular cells, microvilli, and epithelial tight junctions [23,27–29].
Furthermore, Taguchi et al. [26] reported the induction of early-stage nephron structures by reaggregating the fractionated Osr1+ integrin alpha 8 (Itga8)+ Pdgfra− metanephric precursor cell populations. These aggregates were co-cultured with mouse embryonic spinal cord or stimulated with Wnt4 (Fig. 3). The resulting structures contained CDH1+CDH6+_tubular segments and WT1+NPHS1+ podocytes. However, these kidney organoids were identified as immature structures that still expressed nephron progenitor cell markers such as SALL and PAX2. This was attributed to the use of mouse spinal cord in the process. Additionally, the growth factors employed in this protocol were poorly defined for the same reason [30].
The kidney organoid protocols mentioned earlier are primarily concentrated on inducing the posterior intermediate mesoderm. Although numerous protocols utilizing CHIR can be used to effectively differentiate PSC-derived nephron progenitor cells, the absence of vascular networks results in inadequate nephron maturation, as well as the lack of ureteric buds and stromal cell populations.
Manipulation of kidney organoid-derived PSCs for maturation
Although previous protocols have successfully established nephron progenitor organoids, the replication of mature glomerular structures and renal function remains a challenge. To generate more mature kidney organoids, including the glomerular filtration barrier and slit diaphragm, a vascular environment is necessary [31]. PSC-derived kidney organoids were transplanted into the subcapsular spaces of mouse kidneys, where they underwent neovascularization to form the glomerular basement membrane, fenestrated endothelial cells, and podocyte foot processes. Furthermore, in vivo imaging has demonstrated connections between functional glomerular perfusion and conventional vascular networks [30,32,33].
Effective manipulation of mature nephron progenitor cell populations or podocyte organoids has been proposed. These organoids exhibit morphological and functional features, such as primary and foot processes. A protocol has been developed to activate Wnt-induced podocytes derived from human and mouse PSCs, which express key markers of mature podocytes, including synaptopodin, nephrin, podocin, WT1, and actinin-4. Additionally, the induced podocytes demonstrated cytoskeletal rearrangement in response to angiotensin II, and drug uptake was confirmed. The Bonventre group published a study on kidney organoids derived from genome-edited iPSCs using CRISPR/Cas9 knockout technology. In these PODXL-deficient kidney organoids, podocyte-like cells displayed nephron defects, including an almost complete absence of microvilli. This study also showcased disease modeling of cystogenesis in hPSC-derived kidney organoids using biallelic truncating mutations in polycystic kidney disease (PKD) gene 1 (PKD1) or PKD2 [34–37].
The derivation of the anterior intermediate mesoderm gives rise to ureteric bud progenitor cell populations, which form a distinct lineage of kidney organoids. The duration of CHIR treatment for anterior intermediate mesoderm induction (approximately 24 hours) is shorter than that for posterior intermediate mesoderm induction, directing the iPSCs toward the ureteric bud lineage. Ureteric bud organoids exhibit branching morphogenesis and a mature collecting duct system. The isolated T-shaped ureteric bud, derived from PSCs, expresses Proto-oncogene Tyrosine-protein Kinase Receptor (RET). RET is well known for its involvement in the development of ureteric progenitor cells and in expandable branching morphogenesis. The ureteric bud organoid was found to express key regulators of ureteric progenitor cells, including RET, ETS translocation variant 5 (Etv5), SRY-box transcription factor 9, and ureteric bud lineage markers such as Gata3, Pax2, keratin type II cytoskeletal 8 (Krt8), and Cdh1. The transcriptome profiles of ureteric bud organoids, encompassing primary ureteral bud and trunk populations, were analyzed using RNA sequencing to confirm their differentiation potential [38–40].
Published approaches to kidney organoids have successfully established appropriate induction protocols for various renal cell types, which express markers and undergo structural patterning during kidney development. These approaches have also demonstrated the ultrastructural and functional features of the kidney. Single-cell RNA sequencing of kidney organoids, employing reporter-based mapping and barcode sequencing, has revealed broad similarities in genetic screening data between induced kidney organoids and human fetal kidneys. Genetic defects associated with renal disease can be identified through the genetic profiling of kidney organoids, taking into account relevant developmental and functional aspects [41–46].
Generation of higher-order kidney organoid structures
The previously described approaches generated nephron progenitor or ureteric bud cell populations for each renal lineage. In contrast, Taguchi et al. reported the generation of higher-order kidney organoids using lineage-tracing methods in mice. The authors analyzed detailed information regarding gene expression during mouse fetal kidney development from embryonic day 7.5 to 11.5. This approach, employing well-defined growth factor concentrations and suggested treatment durations, resulted in the induction of ureteric and metanephric mesenchymal lineages, respectively. A high concentration of CHIR was used to induce the metanephric mesoderm, while detailed regulation involving retinoic acid, Wnt, and FGF/GDNF induced ureteric bud-like cells; this was followed by the maturation of Wolffian duct progenitor cells. The C-X-C chemokine receptor type 4 (Cxcr4)-/tyrosine-protein kinase (Kit)-positive cell populations were sorted and reaggregated, forming ureteric bud-like structures that expressed ureteric markers, such as Wnt11, RET, Sox9, and cytokeratin 8 (CK8). After inducing ureteric buds and metanephric mesenchyme lineages, respectively, the ureteric bud and metanephric mesenchyme reaggregated with stromal progenitor populations expressing platelet-derived growth factor receptor alpha (Pdgfra), which dissociated from the mouse kidney on embryonic day 11.5 (Fig. 3). These reconstituted kidney organoids improved the branching morphogenesis of the ureteric bud lineage and expressed typical markers, such as Sox9 at the ureteric tips with Gata3 and calbindin 1 (Calb1) in the ureteric epithelium. Reassembled higher-order organoids also mimic the physiological interactions and structural features of human embryonic kidneys [26,47].
The stromal progenitor cell population differentiates into various types of interstitial cells, making it crucial to construct the stroma between the ureteric bud-derived epithelial parenchyma and nephron progenitors. However, due to the nature of kidney organoids, the protocols used in previous studies are insufficient for generating stromal progenitor populations without a vascularization environment. Little and colleagues reported that kidney organoids contained PDGFRA-positive stromal cell populations, such as early mesangial cells, as well as myeloid ecotropic viral insertion site 1 (Meis1)-positive renal interstitial cells [27]. Furthermore, other studies have proposed a co-culture method to enhance glomeruli under vasculature conditioning for stromal cell induction [48–50].
Recently, the Nishinakamura group developed a protocol to generate organotypic “higher-order” kidney organoid structures using PSC-derived renal stromal cell populations. They established dorsoventral patterning of stromal progenitors in the posterior intermediate mesoderm using roundabout guidance receptor 2 (ROBO2) and PDGFRA. The sorted stromal progenitor cell populations were then reaggregated with induced ureteric bud and nephron progenitor populations (Fig. 3). These higher-order organotypic kidney organoids exhibited a more comprehensive structure, characterized by increased ureteric bud branching and stromal cell formation. In comparison to previously reported kidney organoids, the generated kidney rudiments displayed a more intricate glomerular structure due to the presence of various stromal cell types surrounding the glomeruli. Following transplantation into immunodeficient mice, transcriptome profiles were employed to identify each stromal subpopulation, including cortical and medullary stroma, mesangial, renin, and smooth muscle cells [51].
Kidney tubuloids derived from adult stem cells
Both ESC- and iPSC-derived kidney organoids possess the benefit of exhibiting the intricate and organotypic characteristics of the human kidney. Typically, kidney organoids derived from PSCs require 2 to 3 months for complete differentiation. However, they cannot undergo expansion and exhibit inadequate reproduction. To produce kidney organoids of consistent quality, it is essential to consider factors such as the pluripotency states of PSCs, variations in passage at the initial stage, cell viability, and the handling expertise of different researchers.
ASC-derived kidney tubuloids resemble adult kidney tubules, which have a 3D tubular epithelial structure. They are derived from adult kidney tissues or urine samples. Given that they have tubular structures without glomeruli, they are referred to as “tubuloids.” ASCs in kidney tissues and urinary samples are already fully specified to the kidney tubule and can form a cystic, highly polarized epithelial structure within 7 to 14 days. The protocol for ASC-derived tubuloids is relatively simple compared to that for kidney organoids derived from PSCs. A well-defined differentiation medium consists of growth factors and R-spondin–conditioned media, with or without Wnt3a stimulation. Tubuloids maintain genetic stability during long-term culture, as karyotyping reveals a normal number of chromosomes. Moreover, fully differentiated tubuloids can be expanded and cryopreserved. In the tubuloids, immunostaining and sequencing data confirm the renal tubular nature and epithelial structure of the kidney, including the proximal and distal tubules, loop of Henle, and collecting duct epithelium. These tubuloids exhibit transporter activity in a leak-tight formation and a polarized structure in an organ-on-a-chip format. Patient-derived tubuloids are suitable for genome editing and can be employed to model diseases such as hereditary tubulopathies and childhood kidney tumors. Additionally, the tubuloid platform can be utilized for drug uptake transporter assays and the assessment of treatment effectiveness [52–54].
Disease modeling using kidney organoids and tubuloids
Kidney organoids and tubuloids have been utilized in disease modeling to demonstrate renal glomerular and tubular abnormalities. Additionally, kidney organoids have been employed to investigate the function of target genes during kidney development and disease pathogenesis. As mentioned in the section on podocyte-like organoids, CRISPR/Cas9-mediated knockout of PODXL, which encodes the podocyte protein, results in defective junctional organization in PODXL−/− kidney organoid podocytes [36]. Moreover, intraflagellar transport protein 140 (IFT140)-mutated organoids were generated using iPSCs from patients with nephronophthisis-related ciliopathy. The gene-edited proband organoids exhibited shorter, club-shaped primary cilia and abnormal polarity in the renal tubular epithelium. Furthermore, IFT140 gene-corrected organoids were rescued and displayed a normal distribution of IFT components [55]. Little and colleagues proposed that kidney organoids are suitable for podocytopathy modeling because their podocyte-specific gene expression and polarized protein localization are similar to those in conditionally immortalized human podocyte cell lines [56]. A mutation of NPHS1, which encodes nephrin, was found to cause glomerular podocyte abnormalities and slit diaphragm deficits in a kidney organoid model. Organoids derived from patients with nephrotic syndrome were also studied for abnormal slit diaphragm formation and mislocalization of the mutant nephrin protein [57].
Previous research on PKD modeling using kidney organoids demonstrated that the removal of the associated proteins polycystin-1 and -2 led to autosomal dominant PKD (ADPKD)-induced cyst formation in the tubular segment. However, the efficiency of cyst formation was low, with only 6% of the total kidney organoids affected [36]. In suspension culture in which adhesive force had been eliminated to promote more effective cystogenesis, 75% of the PKD−/− organoids treated with forskolin and 8-Br-cAMP developed cysts that were 10 times larger than those in adherent cultures [58]. Additionally, a study investigating the therapeutic effectiveness of tolvaptan, a selective vasopressin-2 receptor antagonist, and CFTR inhibitor 172 on ADPKD patient-derived kidney organoids indicated the potential for developing therapeutic drug targets based on kidney organoid disease models [59].
In vitro drug screening using kidney organoid models
Renal drug transporters are found in the basolateral and apical membranes of proximal renal tubules. These transporters are responsible for the reabsorption and secretion of drug molecules [60]. Previous in vitro studies have utilized primary human renal proximal tubule cells and immortalized kidney cells to assess drug-induced nephrotoxicity. In conjunction with refining mature kidney organoid differentiation protocols, researchers have conducted nephrotoxicity studies on various drug responses using the kidney organoid platform.
The proximal tubule within the glomeruli of kidney organoids has been observed to display functional characteristics, such as megalin-dependent, cubilin-mediated endocytosis. Additionally, the dextran uptake assay has been employed in numerous studies to investigate the functional properties of differentiated kidney organoids [27,29,36,41,49,61,62].
Nephrotoxicants, including various anticancer drugs and antibiotics such as gentamicin and cisplatin, have been assessed for drug-induced renal toxicity using a kidney organoid model. Renal injury markers, including neutrophil gelatinase-associated lipocalin (NGAL) lipocalin and kidney injury molecule 1 (KIM-1), have been found to be upregulated in cases of kidney damage. Treatment with nephrotoxicants resulted in the deterioration of differentiated kidney-like structures and an increase in KIM-1 expression or γ-histone 2AX staining (γH2AX) in glomerulus-like formations [27,29,36]. A range of drugs, encompassing tubular (gentamicin, citrinin, cisplatin, rifampicin, acetaminophen, and ethylene glycol) and glomerular (puromycin and doxorubicin) targets, have been evaluated using kidney organoids [62,63].
In one study, the efficacy of a drug was evaluated using two-dimensional and 3D kidney organoids, as well as on a larger scale in 96-well plates. Exposure to doxorubicin led to the loss of BFP2 signaling and fragmentation of glomerular structures in MAF bZIP transcription factor B (MAFB)-BFP2 glomerular organoids grown in 96-well plates. Additionally, the expression of the apoptosis marker caspase-3 increased, while the MABF-BFP2 level and glomerular size decreased [56].
Using kidney organoids, a high-throughput screening (HTS) platform can be employed to conduct multidimensional phenotypic analyses for optimizing differentiation protocols. Freedman and colleagues demonstrated that the HTS platform could be used to assess the differentiation efficiency of kidney organoids in 96- and 384-well formats. This platform showcased the miniaturization and automation of human kidney organoid differentiation. Although it was a relatively simple step, employing a high concentration of CHIR at 60 hours, the automated induction method generated nephron-like structures with a single organoid in each well. Utilizing this HTS platform, quantitative evaluations of a wide range of CHIR concentrations were conducted, and the relationship between the inducer and differentiation induction efficiency was examined [46].
Conclusion and perspectives
In recent years, progress has been made in developing protocols for kidney organoids derived from PSCs and ASCs. These established protocols have successfully generated complex, multicellular kidney-like structures with renal functions. Single-cell sequencing analyses have shown that the induced kidney organoids possess a profile similar to that of human fetal kidneys. Kidney organoid generation protocols involve the differentiation of metanephric mesenchymal and ureteric bud lineages, as well as more advanced protocols for inducing heterogeneous stromal cell populations. These kidney organoids are utilized to study renal pathogenesis and kidney organogenesis. The recent rapid advancements in the field of renal organoids have led to the development of mature and complex kidney organoids, which contribute to our understanding of renal disease pathogenesis and have potential applications in disease modeling. Furthermore, kidney organoid platforms can be employed for nephrotoxicity and drug efficacy testing, which may help reduce the need for and use of experimental animals [64]. Overall, advances in kidney organoid induction technology, along with the diverse range of applications mentioned above, are expected to drive scientific progress and open up possibilities for transplantable organ applications.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by the National Research Foundation of Korea (2018R1A5A2025079 to H. Y. G.) and by a research grant from the Handok Jeseok Foundation (2021). The funders had no role in the publication decision or manuscript preparation.
Authors’ contributions
Conceptualization: HYG; Funding acquisition: HYG; Investigation: HK, SY, YJC; Visualization: HK, SY, YJC, HYG; Writing–original draft: HK, SY, YJC, HYG; Writing–review & editing: HK, SY, YJC, HYG.
Data availability
None.
Acknowledgements
The authors thank MID (Medical Illustration & Design) for providing excellent support with medical illustration.
