Cryopreservation of engineered tissues and organoids
Article information
Abstract
Although engineering transplantable tissues for clinical applications is difficult, cryopreserving those tissues to create pre-made engineered tissue for emergency transplantation is an even greater challenge. It remains uncertain whether the cryopreservation of engineered tissues will become a reality, but if so, it could have a significant impact on public health by saving lives and simplifying clinical treatment procedures. All in all, the potential implications of this innovation could have far-reaching consequences for the medical field as a whole. This review introduces the basic principles of cryopreservation techniques, including the procedures and components involved. Additionally, a list of its applications to engineered tissue is presented.
Introduction
Organoids are 3-dimensional (3D)-cultured cell constructs that encompass a diverse range of cell types, closely mimicking the structure, organization, and some functions of tissues and organs [1]. Cultured organoids do not grow beyond a few millimeters in size due to limitations to the passive diffusion of oxygen, nutrients, and other soluble substances. Therefore, vascularization is required for sustained growth and maturation to occur [2,3]. Organoids have garnered attention as an alternative to gene therapy and damaged organ transplantation, as they can replicate human physiological functions to a certain extent. By constructing organoid-like structures from patient tissues, organoids enable disease modeling based on the patient's genetic information, gene editing techniques, and high-throughput screening for drug discovery. The prospects for organoids include personalized medicine, potential disease diagnosis, development of disease models, and therapeutic applications [4–6]. The strong demand for a steady supply of organoid cultures arises from a wide range of potential practical applications. Introducing a cryopreservation step into the manufacturing pipeline is expected to broaden the availability of tissue-like systems for academic, research, clinical, or commercial needs. Additionally, the cryopreservation of complex organotypic structures during long-term large-scale culture offers a feasible solution for controlling product quality in a reproducible manner. Therefore, the cryopreservation of organoids necessitates further research and development [7]. Cryopreservation plays a critical role in the preservation of biological materials such as cells, organelles, tissues, and membranes, ensuring their long-term viability and functionality. However, the cryopreservation of tissues and organs presents a complex challenge due to the formation of ice crystals, which can lead to significant cell damage and loss of viability. This review aims to provide a comprehensive overview of the obstacles and approaches involved in achieving successful cryopreservation of organs and tissues. The article focuses on the crucial task of controlling and inhibiting ice crystal formation during the freeze-thaw cycle to minimize detrimental effects on cellular structures. It delves into 2 commonly employed cryopreservation methods—namely, slow freezing and vitrification—and discusses their specific applications for different types of cells. This review underscores the importance of employing cryoprotective agents (CPAs) and selecting appropriate cooling and thawing rates to optimize the cryopreservation process. Furthermore, it highlights the necessity for further research to develop innovative techniques for preserving organs composed of diverse tissue types. Overall, this review emphasizes the significance of cryopreservation as a foundational approach in various biological applications, while elucidating strategies aimed at enhancing the success of organ and tissue cryopreservation.
Cryopreservation methods
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
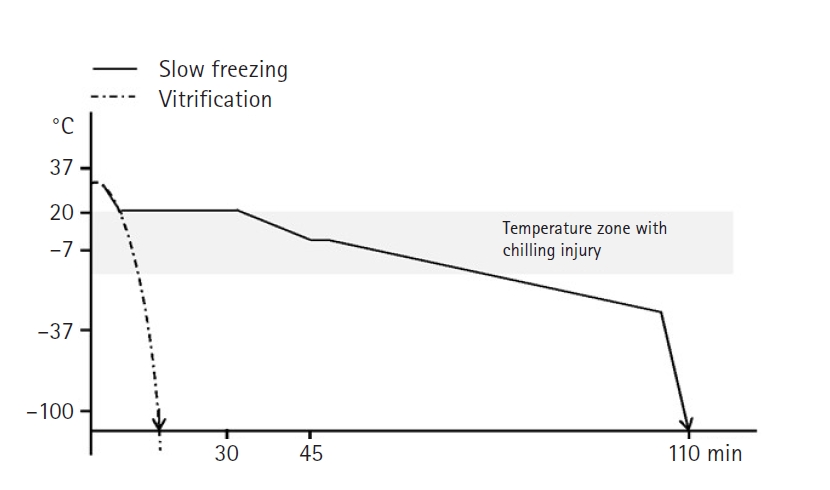
Cryopreservation is a fundamentally important approach for preserving cells, organelles, tissues, membranes, and other organic materials, which can reduce biological activity and provide essential support for a variety of biological applications [8]. The field of cryopreservation has emerged as a practical means of preserving living cells and tissues, and it has grown to find applications across biology and medicine. Cryopreservation offers the potential to provide significant advantages in various therapeutic and medical fields by enabling easy storage of cells, tissues, and organs [9]. However, It has been observed that the cryopreservation of spheroids and small tissue structures is more challenging than the cryopreservation of single cells [10]. This tendency results from the relative cell recovery immediately post-thaw; however, at times, a “positive” recovery outcome, where cells appear viable immediately after thawing, can be achieved. Nonetheless, cellular demise and necrotic activity might be interpreted as cell loss only after some time has passed. Efficiently preserving organs/tissues is a challenging task, and there is a need for research on new techniques for the preservation of various tissues [11]. Cryopreservation can be performed using 2 methods: slow freezing and vitrification. The main differences between the 2 methods relate to the time required and the concentration of CPAs used during the freezing process (Fig. 1) [12]. In the slow freezing method, the sample is gradually cooled at a rate of 0.2ºC to 2ºC per minute until it reaches a final temperature of –196ºC. In contrast, the vitrification method involves rapidly freezing the cells within a few minutes, necessitating higher concentrations of CPAs to achieve faster freezing rates. Multiple studies have reported that slow freezing yielded superior results in liver cells, hematopoietic stem cells, and mesenchymal stem cells (MSCs), while vitrification showed better outcomes in oocytes, pancreatic cells, and embryonic stem cells [13]. To ensure successful preservation, 2 protective measures need to be implemented: the application of a CPA and the selection of an appropriate cooling and thawing rate [6]. Freezing the specimen too quickly results in ice formation, while excessively slow freezing causes cell dehydration and shrinkage. Therefore, the cooling rate should be optimized based on the specific sample type. Researchers have been exploring various strategies, such as incorporating anti-freezing agents, adjusting the thawing temperature, and supplementing with antioxidants, in order to minimize the negative effects of freezing preservation. Therefore, this review aims to provide an overview of the challenges and strategies involved in successful organ/tissue cryopreservation.
1. Slow freezing
Slow freezing is a step-wise programmable freezing method that uses a computerized refrigerator to gradually decrease the temperature of specimens. This method reduces the possibility of intracellular freezing by dehydrating cells [14]. Slow freezing has the disadvantage of taking a long time (2–4 hours), but it has the advantage of minimal cell damage due to the use of low levels of CPAs. Higher concentrations of CPAs enable more effective cellular preservation, but increasing CPA concentrations can also result in cell damage. Cooling rates of approximately 1℃/min are commonly used to avoid damage to cells during slow freezing. According to research, larger cells generally exhibit a slower dehydration process compared to smaller cells, thereby enhancing cell viability when cooled slowly [13]. A comparative study on ovarian tissue cryopreservation methods involving slow freezing and vitrification demonstrated that the slow freezing method is an effective approach for preserving ovarian tissue without significant structural damage [15]. Additionally, evaluating the impact of slow freezing and vitrification on adult testicular tissue revealed that survival was evident with controlled slow freezing, along with the preservation of the integrity and structure of the testicular interstitium, including the supportive Sertoli cells [16]. The use of permeating CPAs enables the agents to enter the cells and protect them during the freezing process. This method is particularly suitable for tissues with larger cells and can be optimized by using permeating CPAs or slowing the cooling rate.
2. Vitrification
Vitrification is a rapid-freezing method that involves pretreatment by immersion in liquid nitrogen. To minimize ice nucleation, cells or tissues are exposed to a high concentration of CPA (typically 40%–60% dimethyl sulfoxide [DMSO]) and then rapidly cooled before being frozen at deep cryogenic temperatures using liquid nitrogen [17]. Typically, achieving this involves utilizing a comparatively high concentration of a CPA (typically 40%–60% DMSO) and/or exceedingly rapid cooling rates (15,000–30,000°C/min) [18]. The main advantage of vitrification is that it significantly reduces the likelihood of freezing damage, leading to a higher cell survival rate. However, it requires good manipulation skills and carries a considerable risk of pathogen infection [19]. In the vitrification process of 3D structural samples such as organoids or tissues, the use of high thermal conductivity metals for freezing is necessary. This enables efficient heat dissipation within the sample, preventing cellular damage due to freeze-thaw cycles. Kitazato BioPharma offers an ovarian tissue vitrification storage device as one of its flagship products [20–22]. Numerous studies have demonstrated that the issue of ice crystal formation can be circumvented by employing ultra-rapid vitrification procedures instead of slow freezing protocols. However, despite the reduced stress imposed on cells by vitrification, trauma to female germ cells remains unavoidable [23,24]. There have been successful cases of cryopreservation and transplantation for tissues and organs that are smaller or structurally simpler [25–27]. However, tissues and organs characterized by complex structures and functions have not yet been successfully cryopreserved and transplanted. This is likely due to the intricate and complex nature of these structures, which must withstand various side effects associated with cryopreservation [28]. To achieve a high survival rate during the cryopreservation of organizational structures, further advances in CPAs and vitrification products are essential.
3. Ultra-vitrification
Ultra-vitrification is an emerging technique that combines the advantages of slow freezing and vitrification. It is increasingly recognized as a promising method for cryopreserving germ cells and other single cells. Researchers have made efforts to achieve rapid cooling rates in cell vitrification by using lower concentrations of CPAs to mitigate the harmful effects of CPA-induced toxicity [29,30]. Consequently, the utilization of ultra-rapid cooling and warming has reduced the toxicity associated with conventional vitrification, primarily caused by high concentrations of toxic CPAs and prolonged exposure. Studies have demonstrated that ultra-vitrification exhibits comparable rates of morphological survival to those achieved with conventional vitrification protocols [31].
Table 1 summarizes the main differences between slow freezing, vitrification, and ultra-vitrification.
Thawing (warming)
The quality of biological samples is influenced not only by the cooling process, but also by the thawing procedure. While single-cell cryopreservation technology has seen success, ensuring the survival of perfectly frozen tissues and organs during thawing remains a challenging endeavor. Thawing following cryopreservation can lead to significant damage due to the formation of ice crystals, a phenomenon known as ice recrystallization. This process involves the growth of large ice crystals during cell and tissue thawing and can result in substantial harm. Furthermore, thawing can cause severe structural damage to cells and tissues, leading to reduced viability, irrespective of the storage and thawing methods used [9,13]. To maximize cell viability, rapid thawing in a 37°C water bath for 90 to 120 seconds is recommended [14,32]. However, when dealing with structures that exhibit large-scale organization, this task becomes notably complex. Achieving uniform heat transfer across the entire structure is a significant challenge. Uneven warming during the cryopreservation process can also create mechanical thermal stress within the tissue, potentially leading to tissue fracturing [20]. The diversity in cell types and densities, along with morphological differences among constituent cells, significantly impacts the thawing and thermal conditions of tissues. Organs and tissues are highly sensitive to cooling and thawing rates. Therefore, it is vital to ensure rapid and even heat conduction during freezing and thawing processes. Biological tissues possess low thermal conductivity and high specific heat, resulting in substantial temperature gradients within the tissue during thawing. Thus, it is essential to employ a rapid and uniform heating technique to prevent potential ice recrystallization and mitigate damage from thermal stress [10]. In addressing these challenges, a technology called nanowarming has been introduced. This method entails the incorporation of biocompatible magnetic nanoparticles alongside or within vitrification solutions, loaded either at the boundary or through perfusion into tissues prior to cryopreservation. Subsequently, radiofrequency fields are employed to stimulate these nanoparticles across the entire tissue, facilitating swift and even warming. This innovative approach holds the potential to uniformly thaw tissues and organs at rates of up to 100°C per minute [33–35]. To determine the optimal temperature changes during cooling and warming for various tissue types, it is imperative to conduct thermodynamic studies tailored to each specific tissue's temperature variations.
Cryoprotective agents
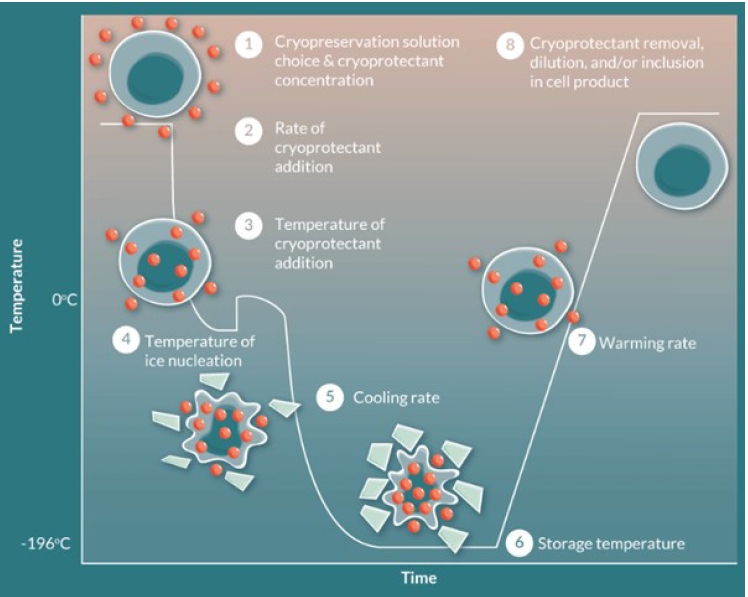
CPAs have been developed to reduce the physical damage caused by ice crystal formation at cryogenic temperatures. Without the assistance of CPAs, cell viability is typically compromised upon exposure to temperatures below 0℃ [13]. Lower temperatures in the cell membrane lead to a reduction in the kinetic energy of phospholipids within the bilayer. Consequently, the fatty acid tails exhibit decreased movement, increased rigidity, and closer clustering. These changes strengthen intermolecular interactions, resulting in reduced membrane fluidity and permeability. As a consequence, essential molecules such as oxygen and glucose may encounter obstacles in crossing the membrane and entering the cell. Moreover, low temperatures impede cellular processes, slowing down cell growth. Prolonged exposure to sub-freezing temperatures can cause the liquid within cells to freeze and form sharp ice crystals, which are capable of piercing the cell membrane and potentially causing cell death [36]. To address these challenges, CPAs have been developed. These compounds aim to protect cells from the detrimental effects of freezing temperatures by minimizing ice crystal formation and reducing the rigidity of the cell membrane. CPAs are incorporated into cryopreservation processes maintain cell viability and functionality (Fig. 2) [37]. The most widely used CPAs, such as DMSO, polyethylene glycol, ethylene glycol, and glycerol, contain permeable components that help regulate osmotic pressure and reduce osmotic damage [38,39]. The penetration of cells needs to be uniform and accomplished without imposing osmotic stresses, as these stresses themselves can be destructive. DMSO, ethanol, and methanol are notable for their rapid and seemingly universal penetration capabilities. However, glycerol has a significant drawback due to its slow movement across membranes that are permeable to it, as well as the large number of tissues that appear to be virtually impermeable to it [40]. In addition to DMSO, low-molecular-weight aprotic synthetic zwitterions have been introduced as a new CPA to improve cryoprotective effects. Synthetic zwitterions, which have various cations (e.g., imidazolium, ammonium, and pyridinium) and anions (e.g., carboxylate and sulfonate) can be cryopreserved and have low cytotoxicity. While cell-permeable natural zwitterions can directly inhibit intracellular ice formation, cell-impermeable synthetic zwitterions indirectly inhibit intracellular ice formation by increasing the osmotic pressure of the freezing medium and dehydrating the cells due to the high osmotic pressure [11]. Long-term exposure to permeable CPAs can cause cell damage. Therefore, it is crucial to develop media without CPAs or with non-toxic CPAs for cryopreservation and storage [41]. These toxic effects can be avoided by using cryopreservation media that minimize DMSO concentration or by rapidly and gently removing DMSO after thawing [42]. Non-permeable CPA substances such as sucrose and trehalose have been widely used as natural CPAs, CPAs for freeze-drying, and stabilizers during dehydration processes [43–48]. The most promising strategy for preserving organized tissues and organs using artificial cryoprotection still revolves around the advancement of non-toxic CPAs that can penetrate rapidly, or finding methods to prevent toxicity or reverse its effects [40].
Cryoinjuries
The cryopreservation process can induce cell death through 3 mechanisms: the formation of extracellular or intracellular ice crystals, membrane damage, and osmotic shock [19]. Cell death in cryopreservation is a physiologically programmed process characterized by controlled mechanisms such as caspase activation, DNA fragmentation, and membrane blebbing. In contrast, necrosis, which falls under the category of cell death pathways, is characterized by organelle and cytoplasmic swelling, followed by the rupture of the plasma membrane and eventual cell lysis [49,50]. In order to minimize cell damage during cryopreservation, scientists have developed CPAs to reduce ice crystal formation, mitigate osmotic shock, and minimize excessive cell dehydration and deformation. Cell damage in biological preservation can arise not only as a result of catastrophic events such as ice crystal formation and osmotic shock, but also due to the development of lethal damage through cell death or necrosis.
1. Osmotic injury
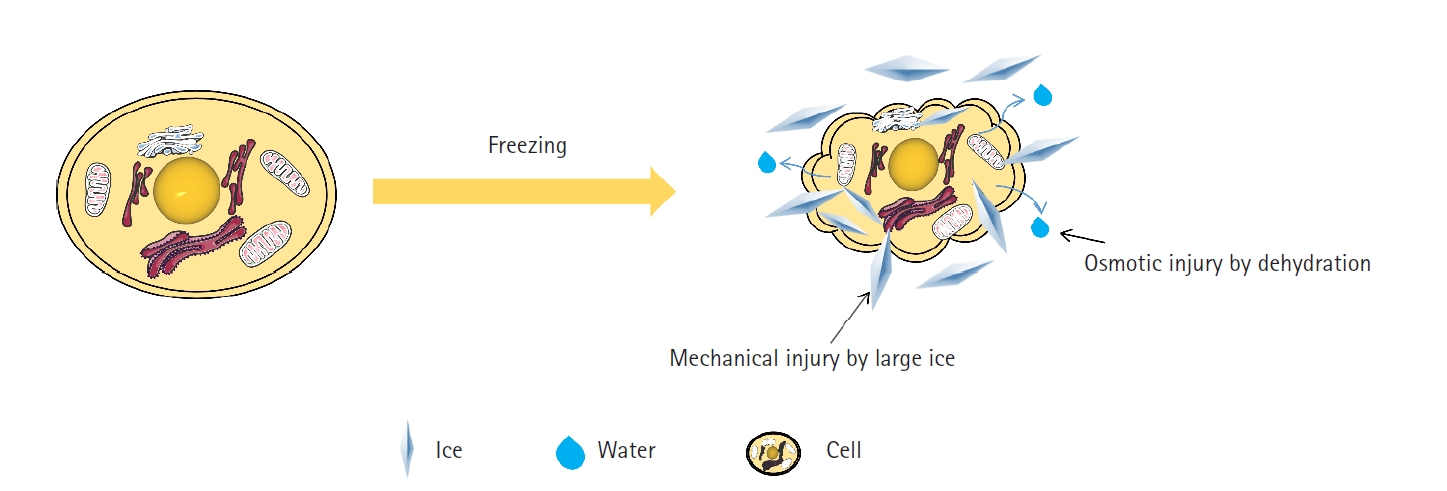
Solute-induced damage occurs due to freezing of the extracellular solution, leading to an increase in solute concentration. CPA and water cause osmotic shock across the cell membrane due to an osmotic imbalance of CPA between the intracellular and extracellular domains. Persistent osmotic shock during the loading and unloading of CPAs is a significant cause of cell damage during cryopreservation [51]. Osmotic damage can disrupt the movement of water molecules across the cell membrane and lead to severe cell dehydration (Fig. 3) [52]. The formation of extracellular ice crystals increases the solute concentration within the unfrozen liquid pockets, creating an osmotic imbalance that hinders the equilibrium of solute concentration between the cell’s interior and the external environment. This imbalance can result in cell membrane leakage and alteration of intracellular water content, thereby interfering with normal cellular function [53,54]. Previous studies have argued that late embryogenesis-abundant proteins trap ions during desiccation, thereby reducing osmotic damage to the membrane. Moreover, in tardigrades, intrinsically disordered proteins not only aid in desiccation tolerance, but also prevent freezing damage, and it is well established that both drying and freezing processes induce osmotic damage [55,56]. Furthermore, it has been observed, using nuclear magnetic resonance techniques, that polyampholytes exhibit significant cryoprotective properties by protecting cell membranes and inhibiting ice recrystallization [57].
2. Dehydration and membrane damage
Membranes undergo severe dehydration during freezing, and one of the critical factors that determine the viability of cells after freezing and thawing is membrane permeability to water. The optimal cooling rate required to minimize cryopreservation damage depends highly on the cell type and is related to the cell's intrinsic membrane permeability to water. At high cooling rates, cell loss is linked to damage by intracellular ice formation, whereas damage at low cooling rates is associated with cell dehydration [58]. The removal of intracellular water increases interactions between molecules. The loss of hydrogen bonding with water in proteins may result in protein aggregation or denaturation by molecular interactions [59]. The protein-protein interactions that result from dehydration may lead to irreversible structural changes [60].
3. Ice formation
When a water droplet comes into contact with a surface below freezing temperature, it can undergo a phase transition from liquid water to solid ice by nucleation and the subsequent growth of ice crystals. Once ice nucleation occurs, the release of the heat of freezing instantly raises the local temperature at the interface between ice and water. The rate at which the ice phase grows depends on the heat transfer from the ice-water interface [61]. Ice crystals inevitably form during the process of cryopreservation, and these crystals can be categorized as either extracellular ice or intracellular ice, depending on their location (Fig. 4) [62]. With an increase in the cooling rate, the moisture within cells cannot flow out rapidly, resulting in the formation of ice crystals inside the cells. Ice crystals that form outside cells both inflict mechanical damage on them and lead to an increase in solute concentration, causing osmotic damage [8,53]. Controlling and inhibiting the formation and growth of ice crystals during freezing and thawing is crucial to minimize cell damage and maintain cell viability [63]. The survival rate of cells decreases when intracellular ice forms due to a higher cooling rate, considering the surface area to volume ratio [64]. To avoid potentially lethal intracellular freezing damage, it is important to maintain osmotic equilibrium between cells and the frozen extracellular environment, which can be achieved by using a low cooling rate [14]. This highlights the significance of preventing intracellular ice formation. The success of cryoprotection relies on effectively preventing intracellular ice formation, which requires careful consideration of water movement across the cell membrane [13]. Combinations of penetrating and non-penetrating CPAs are commonly employed to prevent ice formation. Extracellular ice formation is more feasible than intracellular ice formation. Consequently, the use of non-penetrating CPAs to prevent ice formation reduces the required concentration of penetrating CPAs. Additionally, the use of ice-blocking agents, a special class of non-penetrating molecules, can further aid in cryopreservation by reducing ice formation [65].
4. Hypothermia
Even if ice crystal formation is prevented during cryopreservation, hypothermia can still have detrimental effects on the extracellular matrix (ECM), resulting in its loss and damage [66,67]. Hypothermia negatively affects mitochondrial function, which plays a crucial role in various cellular processes, including ATP synthesis and calcium homeostasis [68]. The various injuries induced by hypothermia are interconnected and mutually reinforcing. The low-temperature state of hypothermia can induce a loss of antioxidant capacity [69], and the increased production of reactive oxygen species (ROS) can damage mitochondria, leading to a decrease in ATP production and acceleration of cellular energy depletion. When ATP levels are depleted, hypoxanthine and xanthine oxidase can accumulate, and their catalytic activity leads to the production of ROS. Additionally, the elevated levels of cytoplasmic Ca2+ induced by hypothermia can activate Ca2+-dependent phospholipases and proteases, leading to membrane depolarization, uncontrolled swelling, and cell necrosis and apoptosis [66]. Loss of ECM due to hypothermia disrupts the chemical interactions and physical support necessary for specific cell types, causing cytoskeletal breakdown, stimulating mitochondrial membrane permeability, and activating caspase family proteases. These proteases induce cell death by degrading numerous proteins in the cytoplasm [70]. ATP depletion, impaired calcium homeostasis, and ROS production are essential contributors to hypothermic damage, as emphasized in several studies [71].
5. Oxidative stress
The intracellular redox state is one factor that can influence the viability of cells during cryopreservation [72]. ROS are highly reactive chemicals formed from diatomic oxygen (O₂). ROS are byproducts of normal oxygen metabolism that play a crucial role in cell signaling and homeostasis and are typically present in low, quiescent levels in normal cells [73]. However, an imbalance between excessive ROS production and cellular defense mechanisms can result in oxidative stress [74]. Cryopreservation can deplete superoxide dismutase, which plays a crucial role in defending against oxidative stress in cells. Another issue arises from the impact on the release of cytochrome c, which mediates the release of pro-apoptotic factors from mitochondria. The activation of this mitochondrial pathway of cell demise is frequently reported as a typical characteristic of cryo-damage [75]. Additionally, cryopreservation-related oxidative stress can result in the generation of elevated levels of O2 radicals due to freeze-thaw stress. ROS generated during this process are known to induce various types of damage, including DNA chain fragmentation, lipid peroxidation, and protein oxidation, among others [76]. ROS-induced damage can have a profound impact on cellular functions and significantly reduce the effectiveness of cryopreservation. Intracellular ROS levels increase significantly during cryopreservation, and many studies suggest that administering certain antioxidant compounds, which are major defense factors against ROS-induced oxidative stress, can have beneficial effects in reducing ROS production [77–79].
6. CPA toxicity-induced injury
To achieve successful cryopreservation, the use of effective CPAs is necessary. CPAs are typically employed to prevent ice formation, which can cause freezing damage to biological tissues when they are cooled, and to ensure the survival of cryopreserved biological materials even after thawing. They are essential during the cryopreservation process. DMSO is a standard CPA widely used for cryopreservation of most cell and tissue types [80]. The toxicity of CPAs varies depending on factors such as temperature, CPA concentration, and exposure time in different types of samples. A CPA can be considered toxic if it damages the cell membrane. Cell membrane toxicity refers to a specific type of toxicity, often observed with DMSO. The cell membrane is composed of a bilayer with hydrophilic polar head groups on the outer and inner surfaces, while hydrophobic fatty acid chains are located in the middle of the membrane. The permeation of molecules through cell membranes is influenced by their lipophilicity—specifically, higher lipophilicity is associated with an increase in permeability. However, larger molecules and those that have the ability to form hydrogen bonds have a lower ability to travel through the cell membrane [81]. CPAs can be categorized into 2 groups based on their ability to penetrate the cell membrane. Penetrating CPAs, which can cross the cell membrane and enter cells, are often used in combination with non-penetrating CPAs, which do not enter cells. This combination is used because ice formation occurs more readily outside cells than inside cells. Understanding the permutations of various CPA combinations can help in understanding the mechanisms of CPA toxicity and identifying better combinations [82]. Various strategies have been attempted to overcome the challenge of removing ice while minimizing toxicity [80]. Therefore, finding the optimal formulation of CPAs is essential for preserving cell viability and function [13,48].
Media supplements
1. Antioxidants
Oxidative stress is a well-documented occurrence in cells during cryopreservation, resulting from an imbalance between oxidation and antioxidation triggered by the generation of ROS under extreme conditions, such as low temperature [83]. Antioxidants can convert ROS into harmless substances, thus regulating oxidative stress. The addition of antioxidants to cryoprotective solutions can improve cryopreservation efficiency, but it should be noted that the misuse of antioxidants can have negative effects. The large amounts of ROS generated during cryopreservation can cause oxidation of proteins, lipids, and nucleic acids, resulting in irreversible cell damage and even death [84]. Given this, a strategy involving the use of both antioxidants and CPAs has been suggested as a way to mitigate oxidative damage during cryopreservation [63,85–87]. However, antioxidants do not exert solely beneficial effects on cryopreservation. According to published research, the use of high concentrations of antioxidants reduces ROS, but negatively impacts the cell's endogenous antifreeze mechanism [85].
2. Anti-apoptosis strategy
While the precise role of cell death in low-temperature damage remains largely unexplored, several indications suggest a correlation between hypothermia and cellular demise [88]. To optimize fertility preservation during cryopreservation, various anti-apoptotic drugs have been proposed, and caspase inhibitors have been hypothesized to provide cryoprotection. Anti-apoptotic agents have been employed during the cryopreservation process to hinder the activation of cell death pathways and subsequently improve tissue survival throughout freezing and transplantation [89]. The inclusion of apoptosis inhibitors in cryopreservation solutions or during cold storage has been demonstrated to improve the viability of multiple cell lines and tissues [90,91]. Furthermore, studies have shown that vascular endothelial growth factor suppresses apoptosis in sinusoidal endothelial cells [92] and granulosa cells [93] induced by cold preservation.
3. Antifreeze proteins
Several species naturally possess a unique defense mechanism against low temperatures by producing a fascinating group of proteins known as antifreeze proteins (AFPs). These AFPs exhibit the remarkable ability to bind to ice, effectively preventing the growth of ice crystals. They can be found in various organisms, including bacteria, fungi, crustaceans, microalgae, insects, and fish, categorized as AFPs type I, II, and III. Different AFPs may possess varying levels of activity and bind to different facets of ice crystals. The inhibition of ice recrystallization by AFPs plays a critical role in safeguarding cellular membranes against freezing-induced damage [94,95]. The intriguing properties of AFPs, including their interaction with cellular membranes and their ability to prevent ice recrystallization, have generated considerable interest in their potential integration into cryopreservation protocols [96]. Thorough testing has been conducted to evaluate the effectiveness of AFPs in cryopreservation. Among these proteins, type III AFP has been the subject of extensive research. However, it has been observed that an elevated concentration of AFPs often leads to a reduction in the post-thaw survival rates of cryopreserved cells. This outcome can be attributed to the formation of detrimental needle-shaped ice crystals at high AFP concentrations, which can infiltrate and damage cells during the freeze-thaw process [97,98] Most cryopreservation studies involving AFPs have focused on samples derived from mammals. The addition of AFPs has consistently demonstrated the ability to enhance post-thaw cell viability, irrespective of the freezing method, storage temperature, or biological sample. Nonetheless, a few reports have indicated that AFP supplementation did not yield beneficial effects [97,99]. The utilization of AFPs in cryopreservation necessitates careful optimization, considering factors such as the specific AFP type, cell type, CPA medium, and storage temperature.
Evaluation criteria for successful cryopreservation of engineered tissues or organoids
Cryopreserved organoids or engineered tissues can have a longer shelf life than other ordinary non-cryopreserved cell-based engineered tissue products. An extended shelf life makes the utilization of medicinal products more convenient, since on-demand availability improves access and allows more rapid clinical treatment. If cell-based engineered tissues are constructed using allogeneic cells, those pre-manufactured tissues can be utilized as a ready-to-use transplantable tissue product. For example, the first U.S. Food and Drug Administration -approved cryopreserved engineered tissue, Dermagraft (Organogenesis Inc., Canton, MA, USA), is a class III medical device approved in the United States in 2001 for the treatment of full-thickness diabetic foot ulcers (DFUs). As cryopreserved engineered tissue, Dermagraft has an extended shelf-life of up to 6 months [100,101] unlike other cell-based tissue products that have a shelf life of only a few days.
Still, cryopreserved tissues pose challenges and disadvantages in terms of handling and transportation. Dermagraft is stored and transported at –75℃±10℃, and exposure to room temperature during transfer should not exceed 15 seconds to avoid compromising cell viability. Upon delivery to the surgical room, the thawed product needs to be washed off before transplantation to remove CPA and bovine serum molecules. Furthermore, the transplantation should proceed within 30 minutes after thawing. Although the challenges posed by cryopreserved tissue may seem difficult and disadvantageous, they could be considered minimal compared to the tremendous advantage of ready-to-use transplantable tissues.
The 6-month shelf life of Dermagraft was determined through an evaluation of its specific functionality—namely, uncompromised cell survival, proliferation tendency, and trophic effects [102]. The evaluation criteria to determine whether cryopreservation of engineered tissues or organoids has been successful might vary depending on the type of tissue and structure. However, there are common essential traits to determine whether cryopreserved tissues are comparable to freshly made tissue.
1. Viability of cryopreserved tissues or organoids
The most significantly compromised trait upon tissue freezing is cell survival, and cell survival/tissue viability is the critical parameter that must be evaluated before even considering the functionality of cryopreserved tissues or organoids. For many different types of engineered tissues, cryopreservation compromises cell survival. Many previous studies have shown survival rates of less than 50% after tissue thawing [102–106].
When advanced therapies based on cellularity are administered for regeneration, severely compromised cell viability after transplantation could be tolerable depending on the mode of action. For example, intravenously injected MSCs cannot survive in the circulation and are almost lost around 48 hours after injection, but they still show a beneficial effect on the host [107,108]. On the contrary, unlike intravenously injected MSCs, engineered tissues need to survive and remain at the transplanted site to perform their intended function. The dividing line that indicates an acceptable level of minimum critical cell viability of cryopreserved tissues has not been clearly drawn. However, a cell survival rate of less than 50% is not desirable in terms of the stability of transplanted tissue and manufacturing efficiency.
After cryopreservation or hypothermic preservation, the viability of tissues and organoids could be influenced by various factors such as the developmental status of organoids [109], freezing methods [110,111], the presence of hydrogel polymers in the cryopreserving medium [112,113], and efficient distribution of cryoprotectants [114].
2. Functionality of cryopreserved tissue
In addition to measurements of cell viability, it is also important to consider cell activity when aiming for successful efficacy. For the engineered tissues and organoids to exert their regenerative function and replicate biological processes, live cells in the tissue structure need to exert their distinctive functions with potentiated cell activity. The ultimate goal of cryopreservation is to maintain the viability and functionality of the engineered tissues and organoids upon thawing, so that they can be utilized for their intended purpose in the future. By evaluating cell viability and tissue regeneration potential, one can ensure that the cells are functioning optimally and that the desired therapeutic outcome is achieved. For example, Dermagraft has demonstrated clinical benefits for patients with chronic DFUs lasting more than 6 weeks in both type I and type II diabetes through randomized controlled trials, which were conducted in 35 centers in the United States [114–118]. The group treated with Dermagraft showed a significantly shorter time to complete wound closure, resulting in a 64% increase in wound healing compared with the non-treated control group. Clinical application of Dermagraft stimulated wound healing for a variety of indications beyond DFUs, including the treatment of dystrophic epidermolysis bullosa [119] and chronic surgical wounds [120].
The functionality deemed most critical varies among different types of organoids according to their purpose. For example, to construct cancer organoids for patient-specific drug screening, cell viability after cryopreservation could be considered as a very important criterion for investigating chemotherapeutic drug responses. However, it is even more important for the drug response of cryopreserved organoids to match the fresh organoids that were constructed with the original tissue. In a previous study, after patient-derived tumor tissues were cryopreserved by conventional slow freezing with DMSO or rapid flash-freezing by submerging in liquid nitrogen for 30 seconds and stored at –80℃ for 6 to 12 months, both groups showed viability comparable to the original fresh tissue after thawing. However, the tissues subjected to DMSO-based slow freezing showed inconsistency in their drug response, while cryopreservation by flash-freezing resulted in a drug response comparable to that of the original tissue [110].
3. Structural integrity
One of the major problems in tissue freezing is that the frozen tissues become solidified and, therefore, can be very fragile and brittle. Careful handling may reduce the risk of tissue fractures. However, both the freezing and thawing processes themselves cause damage to the microstructure of frozen tissues. Ice formation and crystallization during tissue freezing cause biophysical harm [121,122] and thermal stress induces micro-fractures of the tissue structure. Non-uniform temperatures throughout the tissue during tissue warming cause mechanical stress, which leads to tissue deformation. To prevent tissue deformation caused by thermal stress, uniform heating is essential. For samples with high thermal conductivity, conventional cryopreservation causes minimal harm to the structure. However, biological tissues such as engineered tissues or organoids have such low thermal conductivity that conventional thawing protocols create a significant temperature gradient that might cause major damage to the tissue structure. However, the slow cooling method is not free from inducing thermal stress-induced fractures, and even worse, it could compromise cell viability by inducing intracellular ice recrystallization [10]. Although ultra-rapid cooling presents technical difficulties, vitrification is a more favorable technique for the cryopreservation of engineered tissues. Since biological tissues are composed of several different types of cells, each of which exhibits a different critical osmotic tolerance level, scaffold-based engineered tissues composed of a single type of cell might be a less challenging goal. Still, the cryopreservation process needs to be optimized for each particular target cell type.
Factors influencing the cryopreservation of engineered tissues
1. Cryopreservation methods
CPA molecules reduce the damage caused by water crystal formation, which plays a key role in ensuring cell survival during cryopreservation. Therefore, CPA molecules need to be distributed thoroughly throughout the engineered tissues and organoids before freezing. Pretreatment of mouse small intestinal organoids with 5% DMSO for 30 minutes at 4℃ showed a better outcome than observed in tissues cryopreserved with 10% DMSO, exhibiting a survival rate comparable to the unfrozen tissues [113].
For cryopreservation of cell suspensions, both conventional slow freezing and vitrification have been optimized to ensure a high level of cell survival [123]. For engineered tissues or organoids, the vitrification method can be advantageous in achieving a rapid distribution of CPA molecules due to the higher concentration of CPA molecules. However, upon thawing, CPA molecules need to be efficiently removed to secure cell activity in terms of viability and specific function. CPA penetration and the temperature gradient across the tissue structure depend on the size and dimensions of the cryopreserved tissues. Therefore, the size and dimensions of the cryopreserved tissues need to be taken into consideration for optimizing the cryopreservation process. In most cases, multicellular tissues resulted in a better outcome with flash freezing, as shown by the freezing of patient-derived tumor tissue [110] and multicellular human testicular organoids composed of spermatogonial stem cells, Sertoli cells, Leydig cells, and peritubular cells [111].
2. Volume of the cryopreserved tissues
Cells seeded on a 2-dimensional (2D) matrix showed better survival after cryopreservation than cells in suspension [103]. As discussed up to this point, significant challenges lie in the efficient cryopreservation of bulky tissues, in terms of the uniform distribution of CPA molecules and temperature changes. The more solid the tissues are, the more difficult it is to ensure cell viability in tissue construction. The maximum thickness of successfully cryopreserved engineered tissue was found for engineered cartilage, with a thickness of 1.8 mm [124]. A scaffold structure 3D-printed with larger pores between the backbone of the scaffold could facilitate the efficient diffusion of CPA molecules, thereby maximizing the dimensions of cryopreserved tissues.
3. Pre-culture before cryopreservation and cell attachment status
Pre-culturing the engineered tissues before cryopreservation can achieve better conditions and improve cell survival. First, pre-culturing engineered tissue in freezing media containing CPA could facilitate the distribution of CPA molecules throughout the tissue. A few previous reports have shown that pre-culturing engineered tissues in freezing medium for 2 to 4 hours resulted in significantly improved cell survival during short-term culture [103,125]. Although brief exposure to CPAs might not change the cell characteristics, and the reports showed potentiated cell survival after cryopreservation, the recovery of cells needs to be measured in terms of functionality, including their potential for differentiation and tissue regeneration. Using a combination of permeating and non-permeating CPAs can reduce the concentration of toxic CPAs, such as DMSO, resulting in better cell activity after the cells are exposed to freezing medium [126,127]. The reduced toxicity of CPAs can enhance cryopreservation efficiency and cell functionality. However, the cryopreservation process needs to be further optimized according to CPA properties and tissue characteristics. Second, while non-polar CPAs such as DMSO, could degrade the non-polar components of the scaffold materials, resulting in attenuated cell attachment [128,129], pre-culturing the engineered tissue in growth medium can have a beneficial effect by ensuring structural stability through ECM production. ECM molecules not only exhibit a structural role in biological tissues, but also exert beneficial effects by modulating cell behavior, such as cell proliferation, survival, mobility, and differentiation, by providing a source of biochemical signals and a reservoir of growth factors, as well as acting as a substrate. Therefore, pre-incubation is generally beneficial for the viability and functionality of cells after thawing. For example, Dermagraft was manufactured by culturing human fibroblasts in polyglactin mesh to produce collagen, growth factors, and cytokines to achieve optimized cell activity upon thawing [130,131]. However, the abundant secretion of ECM molecules during the pre-culture period complicates the freezing process and results in a negative effect by interfering with the diffusion of CPA molecules. The duration of pre-culture should be optimized according to the cell type and resulting microstructure of the engineered tissue.
4. Biomaterials used for scaffold-based engineered tissues and cell encapsulation
There are inconsistent reports on the comparison of cell recovery between cells in suspension or a 2D monolayer. While some reports have shown that cells attached to a 2D matrix have better survival [103], some reports have shown better cell survival with cell suspensions [127,132]. The general expectation for the cryopreservation of scaffold-based engineered tissues is that CPAs efficiently diffuse to reach the cells seeded on the scaffold, resulting in optimized cell recovery. However, previous reports demonstrating that cells seeded on biomaterials or scaffolds exhibit poor survival indicate that a case-by-case approach is needed to optimize the cryopreservation process for engineered tissues. For example, the components of the scaffold can provide cryoprotective effects. In a study by Gurruchaga et al. [126], incorporating the components of synovial fluid into the scaffold resulted in significantly improved the viability of the engineered tissue after cryopreservation.
Furthermore, some types of hydrogel polymers potentiate the cryoprotective effect. The presence of polyampholytes in cryogenic media resulted in high recovery and viability of HepG2 cell-based liver organoids upon thawing [112]. In another study, the group treated with polyampholytes showed intact membrane morphology compared with the group treated with cryogenic medium without polyampholytes, exhibiting a profile similar to non-cryopreserved, fresh organoids. The presence of antifreeze glycoproteins in the cryogenic medium significantly prolonged the survival of intestine organoids in hypothermic conditions. The storage of immature intestine organoids in hypothermic conditions for 72 hours resulted in only 8% of immature organoids showing normal morphology, with failure to survive upon rewarming. However, in the presence of antifreeze glycoproteins, up to 93% of the organoids showed survival after rewarming [109].
The structure provided by biomaterials also provides a cryoprotective effect. Cell encapsulation with a hydrogel polymer (Fig. 5) was conceived almost a century ago [133], and was first reported by Bisceglie in 1934 [134] for immune-protection of transplantable beta-islet cells. In addition to immune protection, cell encapsulation has been reported to provide cytoprotective effects against harsh environments such as oxidative stress [135–137], attachment-deprived states [138,139], and mechanical stress [140]. Encapsulated cells also demonstrated a cytoprotective effect during cryopreservation [133,141]. When the concentration of penetrating CPA molecules was reduced to attenuate CPA toxicity during vitrification, adipose-derived stem cells showed extremely low viability after freezing and thawing. However, cells encapsulated in hydrogel showed significantly potentiated cell viability with the same composition of CPA molecules (Fig. 6) [142].
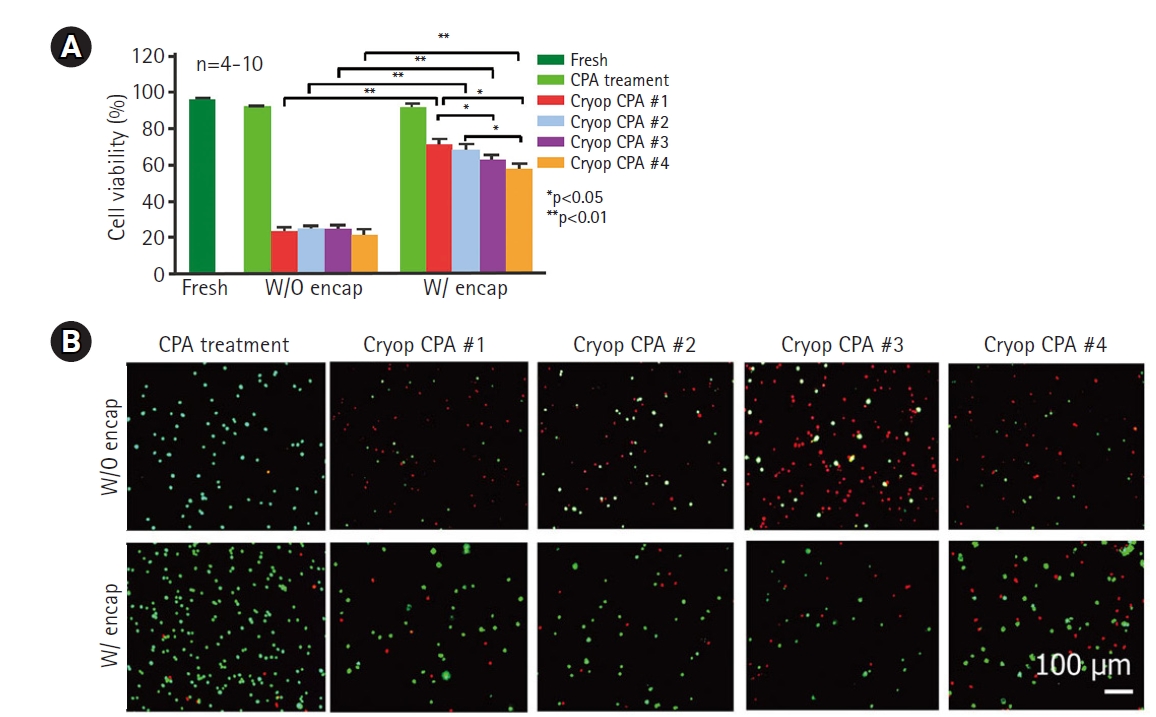
Effect of cell surface micro-encapsulation on cryoprotective effects. Reproduced from Zhao et al. Adv Healthc Mater 2017;6:10.1002/adhm.201700988, with permission [142]. (A) Encapsulated cells showed better cell viability with experimental compositions of CPAs. (B) Live/dead staining results of the experimental groups. (green: live cells, red: dead cells) CPA, cryoprotective agents. Encap, encapsulation. CPA #1: 1 mol L−1 1,2-propanediol (PROH), 1 mol L−1 ethylene glycol (EG), 10% dextran T50, and 1 mol L−1 trehalose; CPA #2: 1 mol L−1 PROH, 1 mol L−1 EG, 10% dextran T50, and 0.5 mol L−1 trehalose; CPA #3: 1 mol L−1 PROH, 1 mol L−1 EG, and 1 mol L−1 trehalose; CPA #4: 1 mol L−1 PROH, 1 mol L−1 EG, and 10% dextran T50. Scale bar=100 μm.
Conclusion
Engineered tissues are being investigated for clinical applications in the treatment of diseases, tissue defects, or injuries. However, there is a time-based discrepancy because it takes time to build transplantable tissue, while some diseases involving fatal tissue failure, such as kidney or pancreas failure, do not allow a long waiting period. Successful cryopreservation of engineered tissues, such as bone, chondrogenic grafts, cardiac constructs, and engineered livers, as well as natural biological tissues, such as ovaries, cartilage, hearts, livers, and blood vessels, can maximize tissue availability. The strategies used for cryopreservation of natural biological tissues also apply to the cryopreservation of engineered tissues. Still, an ideal cryopreservation protocol for engineered tissue should be optimized based on the microstructure of the target tissue type and the metabolic characteristics of the cells it contains. Comparing the parameters of currently optimized cryopreservation requirements shows that cryopreservation of hydrogel-encapsulated cells is similar to that of cell suspension, while scaffold-based tissue-engineered constructs require similar parameters to those of biological tissues and organs [101]. Recent advancements in the field of engineering have opened up new possibilities to enhance cryopreservation technology for improved cell viability. One such technique involves the cell encapsulation technique, which confers cytoprotective effects on the cells. Another promising approach is the modification of cell metabolism by treatment with antioxidant agents, leading to higher cell survival rates. The combination of these and other techniques holds great potential for the future of cryopreservation and its applications in fields such as regenerative medicine and biobanking.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by a grant of the Ministry of Health and Welfare of Korea (HI20C0068), Korean Evaluation Institute of Industrial Technology (KEIT) funded by the Ministry of Trade, Industry, and Energy (20018511), Technology Development Program (S3307363) funded by the Ministry of Small-to-Medium sized Enterprises (SMEs) and Startups of Korea, and the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science & ICT (2021R1A2C2010829). All the funding sources had no involvement in the study, writing of the manuscript, or in the decision to submit the manuscript for publication.
Data availability
All data are available in the main text.