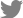|
 |
| Organoid > Volume 3; 2023 > Article |
|
Abstract
NOTES
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number: NRF-2021R1F1A1062027 and RS-2023-00209725), and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0235).
Fig. 1.
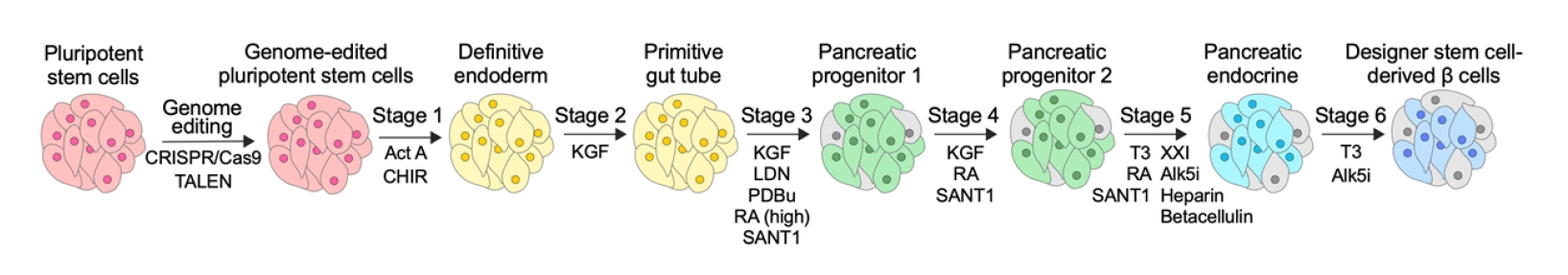
Fig. 2.
Fig. 3.

Table 1.
| Genome-editing strategies and target cells | Editing machinery | Notes | Ref. |
|---|---|---|---|
| Genome editing for improving stem cell-derived β-cell therapeutics | |||
| Knock-in of an EGFP reporter into the C-terminus of PDX1 in iPSCs | SpCas9-mediated knock-in by HDR | The editing method allowed the facile isolation of insulin-positive β cells after differentiation. | [43] |
| Knock-in of a GFP sequence downstream of the INS promoter in iPSCs | SpCas9-mediated knock-in by HDR | Insulin-positive cells could be spotted from a cell mixture. | [44] |
| Knock-out of SLC30A8 in ESCs | SpCas9-mediated knock-out | ZnT8 loss-of-function improved GSIS in SC-β cells. It also endowed the cells with resistance to lipotoxicity- and glucotoxicity-induced cell death. | [49] |
| Knock-out of ZNF148 in ESCs | SpCas9-mediated knock-out | ZnF148 loss-of-function improved insulin secretion by modulating proteins involved in insulin trafficking and exocytosis. | [48] |
| Knock-out of B2M and CIITA, overexpression of CD47 in iPSCs | SpCas9-mediated knock-out, viral delivery of CD47 | The hypoimmune SC-β cells survived long-term in immunocompetent mice and non-human primates. | [54] |
| Knock-out of CIITA and all classical HLA class I genes except HLA-A2, knock-in of luciferase at AAVS1 in ESCs | SpCas9-mediated multi-step gene knock-out, TALEN-mediated knock-in by HDR | The resulting SC-β cells were less immunogenic, allowing long-term survival in a mouse model. Luciferase transgene allowed facile in vivo monitoring of the transplant. | [50] |
| Knock-out of B2M, knock-in of 3 cytokines (IL-2 N88D mutein, TGF-β, and IL-10) at the GAPDH locus of hESCs | SpCas9-mediated knock-out, SpCas9-mediated knock-in by HDR | The B2M knock-out SC-β cells displayed varying success of immune evasion in vivo. Cytokine knock-in was a complementary strategy for allograft survival. | [56] |
| Knock-out of B2M, and knock-in of doxycycline-inducible PD-L1 at AAVS1 locus of ESCs | SpCas9-mediated knock-out, TALEN-mediated knock-in by HDR | The resulting SC-β cells displayed reduced stimulation of diabetogenic CD8 T cells. | [47] |
| Knock-out of B2M in iPSCs | SpCas9-mediated knock-out | Decreased expression of HLA class I on SC-β cells resulted in reduced T-cell activation. | [59] |
| Knock-in of a GFP reporter in the middle of CXCL10 to disrupt the gene in ESCs | SpCas9-mediated knock-in by HDR | Knock-out improved SC-β cell survival against allo-rejection in a mouse model. | [60] |
| Knock-out of RNLS in iPSCs | SpCas9-mediated small deletion by simultaneously delivering 2 gRNAs | Knock-out protected SC-β cells from ER stress and apoptosis. | [61] |
| Genome editing of stem cell-derived β cells for diabetes modeling and patient-specific therapies | |||
| Correction of diabetes-inducing INS point mutations (C96R and C109Y) in patient-derived iPSCs | SpCas9-mediated knock-in by HDR | The mutant SC-β cells displayed reduced insulin secretion, increased ER stress and reduced proliferation, although apoptosis was not promoted. | [65] |
| Correction of diabetes-inducing INS intronic mutation in patient-derived iPSCs | SpCas9-mediated knock-in by HDR | The mutation resulted in the production of splicing variant of insulin mRNA, leading to decreased insulin production. | [66] |
| Correction of a congenital hyperinsulinism-inducing mutation (V187D) of ABCC8 in patient-derived iPSCs | SpCas9-mediated knock-in by HDR | SC-β cells mutated in the SUR1 subunit of KATP-channel recapitulated the phenotype observed in the disease. | [67] |
| Introduction of mutations at PDX1, or generation of PDX1 haploinsufficient models in iPSCs | SpCas9-mediated knock-in by HDR, SpCas9-mediated knock-out | PDX1 mutations impaired β-cell differentiation and functions. | [68] |
| Introduction of heterozygous 4 bp duplication in GATA6 and GATA6-related non-coding SNP in ESCs | SpCas9-mediated knock-in by HDR | GATA6 haploinsufficiency impaired β-cell development. The non-coding SNP lowered GATA6 expression to prevent β-cell development. | [69] |
| Heterozygous and homozygous knock-out of GATA6 in ESCs | SpCas9-mediated knock-out | GATA6 haploinsufficiency led to impaired formation of glucose-responsive SC-β cells. | [70] |
| Knock-out of CDKAL1, KCNJ11, or KCNQ1 in ESCs | SpCas9-mediated knock-out | GWAS-identified gene variants were studied. Disrupting each of them led to impaired GSIS in SC-β cells. CDKAL1-defective cells were hypersensitive to glucolipotoxicity. Drug screening for CDKAL1 knock-out SC-β cells identified a small-molecule enhancer of the cell function. | [71] |
| Knock-out of HNF1A in ESCs | SpCas9-mediated knock-out | Disruption of HNF1A led to the abnormal expression of genes related to β-cell function and development. SC-β cells allowed the study of human β-cell biology distinct from rodent cells. | [72] |
| Correction of diabetes-causing INS variants in patient-derived iPSCs | SpCas9-mediated knock-in by HDR | Gene correction restored insulin production and secretion in SC-β cells. The corrected cells restored blood glucose homeostasis in a diabetic mouse model. | [74] |
| Correction of diabetes-inducing WFS1 variants in patient-derived iPSCs | SpCas9-mediated knock-in by HDR | The corrected SC-β cells exhibited robust insulin secretion and reversed diabetes in a mouse model. | [75] |
| Genome editing in primary islets | |||
| Knock-out of PDX1 and KCNJ11 in primary human islets | SpCas9-mediated knock-out | Regulation and function of primary β cells were impaired by knock-out. | [76] |
| Small deletion at the non-coding KCNJ11-ABCC8 or SIX2-SIX3 locus in primary human islets | SpCas9-mediated small deletion by simultaneously delivering 2 gRNAs | β-cell function was impaired by editing. This study demonstrated the roles of variants located at non-coding regulatory elements. | [76] |
| Knock-out of B2M and CIITA, overexpression of CD47 in primary human islets | SpCas9-mediated knock-out, viral delivery of CD47 | The engineered cells survived in an immunocompetent mice model and reversed hyperglycemia. | [54,77] |
Ref., reference; EGFP, enhanced green fluorescent protein; PDX1, pancreas/duodenum homeobox protein 1; iPSC, induced pluripotent stem cell; HDR, homology-directed repair; ESC, embryonic stem cell; GSIS, glucose-stimulated insulin secretion; SC-β cell, stem cell-derived β cell; HLA, human leukocyte antigen; TALEN, transcription activator-like effector nuclease; IL, interleukin; TGF, transforming growth factor; hESC, human embryonic stem cell; PD-L1, programmed death ligand 1; CXCL10, chemokine ligand 10; gRNA, guide RNA; ER, endoplasmic reticulum; SUR1, sulfonylurea receptor 1; SNP, single-nucleotide polymorphism; GWAS, genome-wide association study.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 979 View
- 23 Download
- ORCID iDs
-
Seeun Jang

https://orcid.org/0000-0002-6759-0864Siyoon Shin

https://orcid.org/0000-0002-0325-2700Yujin Jeong

https://orcid.org/0009-0001-6669-5285Donghyun Lim

https://orcid.org/0000-0002-6070-198X - Related articles
-
Engineered adipose tissue platforms: recent breakthroughs and future perspectives2023 ;3()




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print