Natural biopolymer-based hydrogels as designer matrices for organoid cultures
Article information
Abstract
Matrigel, a mouse sarcoma-derived extract, is considered the gold standard for organoid cultures. However, it has several drawbacks, including inconsistent and ill-defined composition, varying quality between batches, and potential cancer-related health risks. These factors highlight the need to develop chemically defined alternatives to Matrigel. Natural biopolymers derived from living organisms have emerged as promising substitutes capable of creating chemically defined extracellular matrix (ECM)-mimicking materials to support organoids in a 3-dimensional (3D) environment. This article provides an overview of natural biopolymeric hydrogel-based bioengineering approaches for constructing 3D matrices resembling artificial ECM for organoid cultures. It discusses the latest developments in utilizing natural biopolymers to direct the growth, differentiation, and maturation of organoids, along with their translational applications in the fields of bioengineering and biomedicine. Additionally, the article offers perspectives on multidisciplinary research on natural biopolymer-based hydrogels for more practical applications as next-generation matrices for organoid cultures.
Introduction
Organoids are 3-dimensional (3D) mini-organs formed by the self-organization of stem cells, progenitor cells, or tissue fragments, in the presence of biophysical and biochemical signals that simulate the corresponding organ’s in vivo milieu [1,2]. Their tissue-specific structural and functional characteristics, along with their multicellular complexity, provide a potent platform for advancing organ developmental research, disease modeling, drug screening, and tissue engineering [3–5]. Most organoid culture systems have relied heavily on Matrigel, a basement membrane extract produced from Engelbreth-Holm-Swarm mouse sarcoma [6]. Although Matrigel provides a highly functional matrix for cell proliferation and differentiation due to its remarkable stem cell niche signaling properties and tissue-like mechanical properties [7], its heterogeneity, batch-to-batch variation, and ill-defined composition lead to uncontrollable microenvironments and thus poor reproducibility of organoids [8]. Furthermore, the mouse tumor origin of Matrigel limits its use for therapeutic transplantation in vivo due to potential risks of immunogenicity and carcinogenicity [9]. A chemically defined extracellular matrix (ECM)-mimicking material that would allow the precise modulation of the physical and biochemical properties of cellular microenvironments and thus guarantees more consistent generation of organoids is urgently needed to achieve downstream translational applications such as drug screening, tissue engineering, and personalized medicine.
Natural biopolymers, which are biomacromolecules sourced from living organisms such as bacteria, plants, and animals, have become increasingly popular in the creation of hydrogels that mimic the ECM. These hydrogels provide three-dimensional support for organoids. This popularity is due to the molecular structures of natural biopolymers, which closely resemble the ECM, as well as their superior biocompatibility and biodegradability [10–13]. The high content of functional groups (for example, hydroxyl, amino, and carboxylic acid groups) in natural biopolymers allows for the easy impartation of desired bioactivities to cellular microenvironments [14]. This is particularly beneficial when customizing the microenvironmental cues of an artificial 3D matrix. In addition, the abundance of biopolymers in the natural world and their ability to be produced in a cost-effective manner make them highly attractive for organoid cultures. These cultures can be utilized in various industrial fields, ranging from biomedicine (including regenerative therapy, biobanks, and personalized medicine), to foods, pharmaceuticals, and cosmetics.
In this study, we present an overview of cutting-edge natural biopolymer-based hydrogels as a bioengineered matrix for organoid culture. First, we describe the important biochemical properties of natural biopolymers for the creation of hydrogels (Table 1). Next, we present recent advances in organoid culturing in (1) polysaccharide- and (2) protein-based hydrogels. Finally, we examine the existing problems with these natural biopolymeric hydrogels for artificial ECM engineering, as well as future views and prospects.
Preparation of natural biopolymer-based hydrogels
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
Natural biopolymer-based hydrogels have been evaluated as 3D matrices for cell culture due to their ability to deliver superior nutrition to cells, protect cells and delicate medications, and have inherent biocompatibility [15]. The abundance of hydrophilic groups in the backbone of these hydrogels contributes to the matrices’ high water absorption capacity, which is beneficial for cell growth [16]. The formation of complex structures, along with the enhancement of mechanical properties and stability in physiological environments, is achieved through inter/intramolecular crosslinking within the biopolymeric chain. This crosslinking responds to changes in environmental conditions such as pH and temperature, or the introduction of a crosslinking agent like a chemical crosslinker [17–21]. However, physical or chemical crosslinking can lead to a reduction in the availability of functional groups in biopolymers and poorer degradability [22]. Therefore, an effective crosslinking method is crucial for producing hydrogels intended for successful organoid applications (Fig. 1).
Biopolymers can self-assemble into aggregates through reversible physical interactions such as hydrogen bonding, electrostatic interactions, hydrophobic interactions, or host-guest interactions [23–27]. This process allows the creation of hydrogels under mild conditions without the need for crosslinking agents, which is beneficial for encapsulating cells or biomolecules, as it reduces the risk of unwanted interactions with bioactive agents [28]. Physically crosslinked biopolymeric hydrogels are often sensitive to changes in environmental factors such as pH, temperature, or ionic strength, allowing for variations in hydrogel matrix mechanics [29]. The reversible nature of physically crosslinked hydrogels allows for dynamic behaviors such as shear-thinning or self-healing, as well as native ECM-like physicochemical properties, which offer bioengineering options for enhanced organoid formation [30].
Covalently crosslinked biopolymeric hydrogels, which are produced by covalent bonds between polymer chains (e.g., thiol-ene click chemistry and Schiff’s base reaction), have improved mechanical properties in vivo due to their relatively robust gel networks compared to those formed by physical interactions [31]. The rapid gelation period of these hydrogels, typically less than 10 minutes, is achieved through strong covalent bonding under physiologically moderate conditions. This has led to a particular interest in enzymatic crosslinking and photo-crosslinking for the in situ formation of hydrogel matrices [32,33]. Covalently crosslinked hydrogels, in general, act as linearly elastic materials, and organoid morphogenesis may be physically impeded owing to the poor dissipation of significant compressive pressures during colony expansion [34,35]. To circumvent the irreversible nature of covalent bonding, which causes the progressive softening of covalently crosslinked hydrogels, many techniques for enzymatic degradation [36–38], photo-responsive degradation [39], and passive hydrolysis [34,40] have been proposed.
Polysaccharide-based hydrogels
Polysaccharides are polymeric carbohydrates that consist of multiple monosaccharide units covalently linked by glycosidic bonds [41,42]. Many polysaccharides possess ionizable functional groups, such as amines (-NH3+) in chitosan and carboxylates (-COO-) in hyaluronic acid (HA), and the distribution of those functional groups determines their charge density in different pH environments [43]. The physicochemical properties of polysaccharides can be modified through physical, chemical, and enzymatic processes [44]. Hydrogels based on polysaccharides have recently gained attention as platforms for culturing and/or delivering organoids (Table 2) due to their high water-retaining capacity, biocompatibility, and biodegradability [45–59].
1. Alginate-based hydrogels
Alginate is a linear polysaccharide composed of negatively charged 1,4-linked β-D-mannuronic acid (M-block) and α-L-guluronic acid (G-block) units (Fig. 2A). It is obtained from brown algae through alkali treatment [47,60]. This polysaccharide, approved by the Food and Drug Administration, has garnered significant attention for cell encapsulation techniques due to its low toxicity and ease of manipulation [60,61]. Alginate offers several advantages for organoid culture, including cost-effectiveness, the ability to modulate physical and biochemical properties [62,63], and its viscoelastic nature [64].
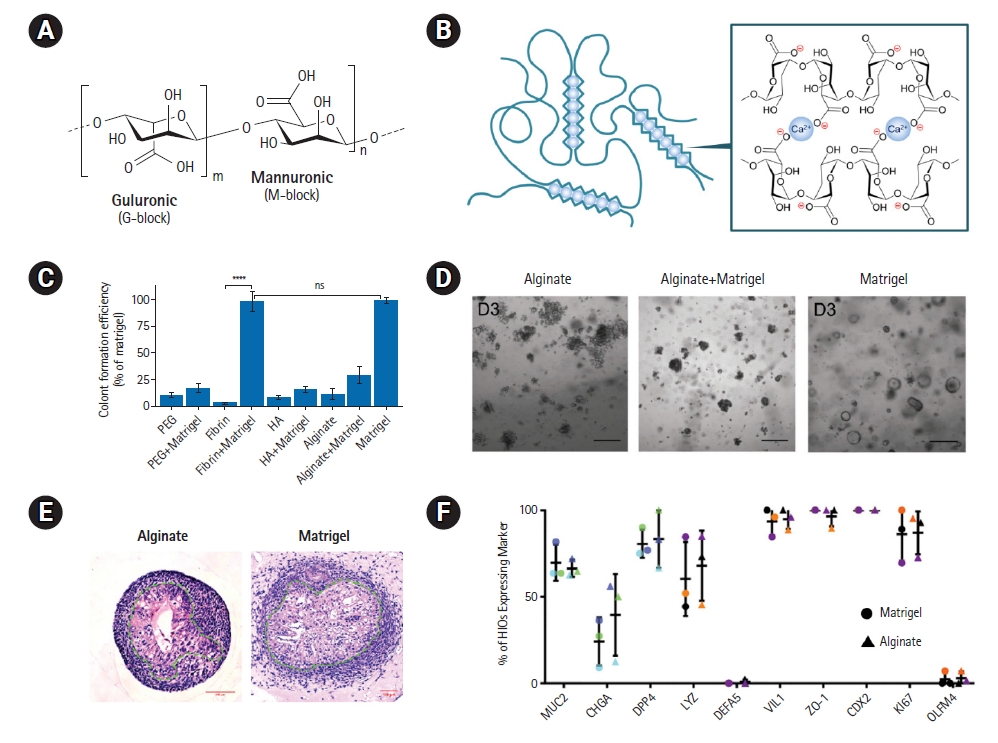
Alginate-based hydrogels for intestinal organoid culture. (A) Chemical structure of alginate. (B) Schematic representation of Ca2+-based ionic crosslinking of alginate. Reproduced from Jo and Lee. Small 2020;16:e1903736, with permission from John Wiley and Sons [10]. (C) Colony formation efficiency of mouse small intestinal stem cells in various hydrogel backbones with or without Matrigel supplementation [48]. (D) Bright-field images of the cultures after 3 days of culture in different hydrogels and Matrigel. Scale bars: 200 μm. Reproduced and slightly modified from Broguiere et al. Adv Mater 2018;30:e1801621, with permission from John Wiley and Sons [48]. (E) Hematoxylin and eosin staining of intestinal organoids cultured in Ca-alginate hydrogel and Matrigel for 28 days. Dashed lines outline the epithelium. (F) Frequency of mature cell type differentiation in Ca-alginate hydrogel and Matrigel. Reproduced and slightly modified from Capeling et al. Stem Cell Rep 2019;12:381–94, with permission from Cell Press [47].
The addition of multivalent cations, with Ca2+ being the most commonly used, enables the rapid gelation of alginate through ionic crosslinking under mild conditions (Fig. 2B) [10,65]. Calcium-crosslinked alginate hydrogels (Ca-alginate hydrogels) have been explored for growing mouse small intestinal stem cell-derived intestinal organoids [48]. However, the colony formation efficiency of mouse small intestinal stem cells within the Ca-alginate hydrogel was significantly lower compared to the counterpart grown in Matrigel (Fig. 2C and 2D). Conversely, another study has proposed that Ca-alginate hydrogel can support the growth and development of human intestinal organoids both in vitro and in vivo, despite its lack of cell adhesive properties (Fig. 2E and 2F) [47]. By culturing human pluripotent stem cell (PSC)-derived hindgut spheroids within a Ca-alginate hydrogel with an appropriate level of stiffness for approximately 30 days, the researchers were able to generate intestinal organoids that closely resembled Matrigel-grown organoids. Moreover, the resulting Ca-alginate hydrogel-grown intestinal organoids exhibited engraftment and maturation after transplantation in vivo to a similar extent as Matrigel-grown organoids, suggesting the potential applicability of Ca-alginate hydrogel as an alternative matrix to Matrigel for culturing intestinal organoids.
Alginate’s inertness and biostability enable cell-specific spatiotemporal imaging and tracking of cells trapped inside an alginate hydrogel for lengthy periods of time [66]. In general, embedding organoids in 3D hydrogels reduces nutrient delivery during conventional static culture because the gel matrix functions as an additional barrier to solute diffusion, making the culture of organoids with high metabolic activity, such as islets, highly unstable for maintaining long-term cell viability and function [66]. However, the continuous dynamic culture of human and rodent pancreatic islets within a 3D alginate hydrogel gelled by BaCl2 solution allowed for the elucidation of complex islet physiological and pathophysiological processes via optical assessment and functional assays using a microphysiological system [49].
Alginate beads were crosslinked using BaCl2 and functionalized with type I collagen, in order to build a platform for disease modeling and medication development for lung illnesses such as idiopathic pulmonary fibrosis [50]. Culture of fetal lung fibroblasts with functionalized alginate beads resulted in the production of cohesive organoids as a consequence of cellular adhesion to the bead surface and subsequent cellular growth and contraction, enabling the formation of self-assembled human lung tissue encompassing numerous cell types.
Alginate modification enables cation-free crosslinking of hydrogels, which may alter their mechanical characteristics and enhance bioactivity [47,67]. The covalent modification of alginate with norbornene (NB-alginate) results in a UV-crosslinkable hydrogel through thiol-ene chemistry, allowing the creation of an ECM-like environment that allows the unhindered passage of most nutrients in lower concentrations of hydrogel [51]. When compared to a standard culture technique on the air-liquid interface, simple encapsulation of kidney organoids within the NB-alginate hydrogel resulted in lower expression of aberrant type 1a1 collagen, with no alterations in organoid structural shape. This synthetic microenvironment, which replicates the in vivo circumstances of the growing kidney, has shown the potential for producing organoids for therapeutic applications.
Furthermore, the potential and relevance of modifying hydrogel characteristics to regulate kidney organoids have been demonstrated using oxidized alginate hydrogels created by imine-type dynamic covalent crosslinking [52]. Kidney organoids were encapsulated in three different stiffness levels (0.1-20 kPa) of hydrogels and two soft hydrogels (0.1 kPa) with adjustable stress relaxation, following induced PSC (iPSC) differentiation (7 days) and air-liquid interface culture (14 days). Kidney organoids grown in soft, rapidly relaxing hydrogels showed a higher degree of maturity in terms of renal structure formation and the expression of epithelial-mesenchymal transition markers, compared to those grown in stiffer or slow-relaxing hydrogels.
2. Hyaluronic acid-based hydrogels
The negatively charged polysaccharide HA is composed of D-glucuronic acid and N-acetyl-D-glucosamine. Because of its capacity to bind with transmembrane receptors (e.g., CD44, CD54, and CD168), HA, a non-sulfated glycosaminoglycan (GAG) abundantly found in the ECM, is implicated in different signaling cascades that impact cell attachment, migration, proliferation, and morphogenesis [68]. HA may be used to create organoid microenvironments because of its high bioactivity; however, its poor degradation rates and unfavorable mechanical properties make it difficult to apply alone in hard tissue [69]. Hybrid hydrogels formed by incorporating HA into enzymatically crosslinked poly(ethylene glycol) (PEG) matrices demonstrated the ability to maintain, expand, or differentiate human bone marrow-derived stromal cells and human hematopoietic stem cells in vitro, eventually generating bone marrow organoids [53]. Furthermore, another HA/chitosan hybrid hydrogel has been reported to promote cerebral organoid development by iPSC culture in Essential 8 (E8) media without the inclusion of neural induction components [54]. Within 10 to 14 days of culture, iPSCs encapsulated in the HA-chitosan hydrogel showed morphological features of cerebral organoids and growth up to 3 mm in the greatest dimension at day 28, while exhibiting specific behaviors of early corticogenesis (e.g., neural rosette and neural tube-like structures).
3. Heparin-based hydrogels
Heparin is a negatively charged polysaccharide composed of L-iduronic acid and D-glucosamine repeats [70]. This highly sulfated GAG molecule has been shown to regulate cell signaling by sequestering heparin-binding domain-associated growth factors [71], as well as blocking coagulation and thrombosis [72]. Heparin’s capacity to bind and stabilize numerous growth factors and proteins, in particular, makes it useful as a building element of 3D matrices for organoid cultures [73,74]. The use of a matrix metalloproteinase-cleavable peptide linker to crosslink heparin hydrogel with 4-armed PEG was shown to stimulate the morphogenesis of proximal tubule epithelial cells (HK-2) into physiologically sized tubule structures [55]. The resultant tubules demonstrated the form and function of the in vivo renal proximal tubule and responded to nephrotoxins, demonstrating their potential for disease modeling and medication toxicity research. This heparin hydrogel was also used to cultivate multicellular polarized mammary epithelial organoids [56]. Human mammary epithelial cells immersed in heparin-based hydrogel demonstrated laminin secretion and organization into basement membrane-like assemblies, enhancing integrin signaling and encouraging the creation of polarized acini.
4. Cellulose-based hydrogels
Cellulose is a plant-derived structural polysaccharide that is separated into nanofibers with diameters ranging from 2 to 60 nm [75,76]. The oxidation mediated by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) enables the conversion of cellulose strands’ main alcohol groups into negatively charged carboxyl groups (Fig. 3A) [77]. When compared to Matrigel, the resulting cellulose nanofibrils (CNFs) form hydrogels with remarkable mechanical properties, such as rapid self-healing and shear-thinning behavior, supporting the differentiation of liver organoids, while exhibiting comparable or even superior levels of hepatic gene expression, hepatocyte function and organoid polarization [57]. The functionalization of oxidized CNFs with a fibronectin-derived cell adhesion moiety, RGD peptide, has shown promise in improving cellular contact between organoids and the cellulose backbone (Fig. 3B and 3C) [58]. Ca2+-mediated ionic crosslinking between CNF carboxyl groups created a milieu conductive to the development and budding of tiny intestine organoids. Furthermore, TEMPO-periodate oxidation has been shown to allow the incorporation of a greater number of carboxyl groups into the cellulose molecule [78], resulting in the functionalization of CNFs with more RGD peptides than CNFs treated alone with TEMPO-mediated oxidation [59]. Mg2+-generated RGD-grafted CNF-based hydrogel-based cationic crosslinking aided in the development of intestinal organoids, while also enabling their long-term culture following passage.
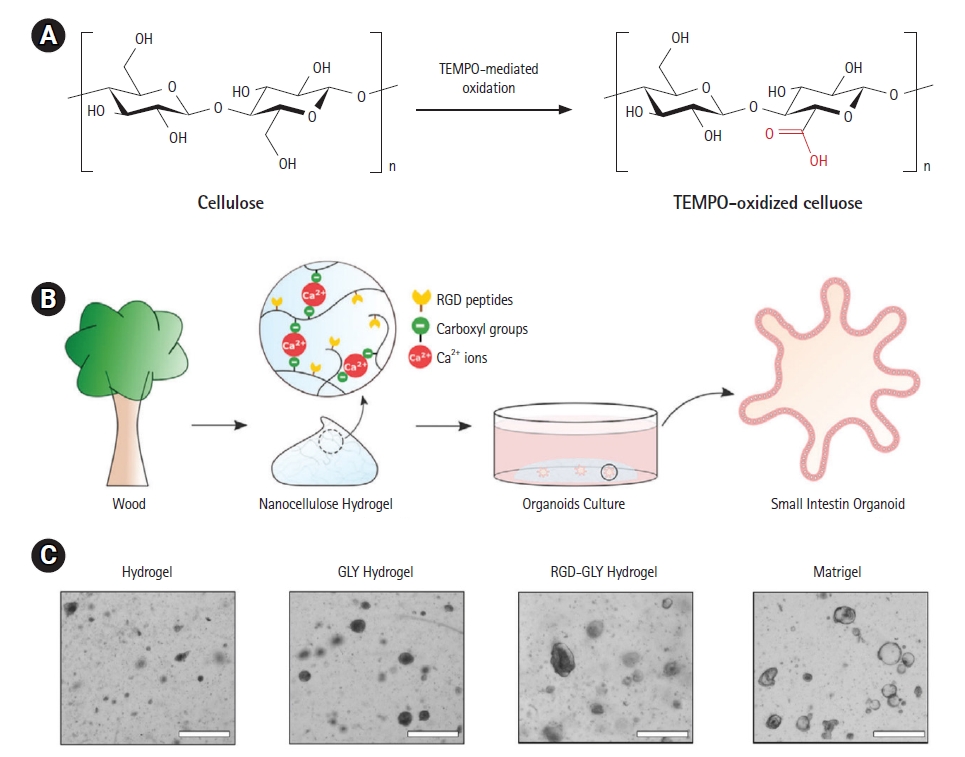
Cellulose-based hydrogels for culture of intestinal organoids. (A) Schematic description of TEMPO-mediated oxidation of cellulose. (B) Scheme of small intestinal organoids cultured in an oxidized cellulose nanofiber (CNF)-based hydrogel [58]. (C) Intestinal organoids cultured in oxidized CNF hydrogels. Cystic organoids are generated only upon the addition of glycine (GLY). The growth of organoids is sustained in the hydrogel of CNFs functionalized with RGD peptide. Scale bars: 100 μm. Reproduced from Curvello et al. Adv Sci (Weinh) 2020;8:2002135, with permission from John Wiley and Sons [58].
Protein-based hydrogels
Proteins are biomacromolecules composed of numerous amino acids linked by peptide bonds [79,80]. They serve as the essential building blocks for highly structured systems that underpin life's critical functions [10]. The sequence of amino acids in a polypeptide chain dictates the protein's physicochemical properties, such as molecular weight, shape, and hydrophobicity, as well as its biological characteristics [43,79,80]. The process of proteins unfolding and refolding mechanically results in inherent viscoelasticity, akin to that of the ECM [81]. The limitless design possibilities and diverse functions of proteins, coupled with their excellent biocompatibility and biodegradability [82], make them particularly attractive for creating hydrogel matrices for organoid cultures (Table 3) [48,83–97].
1. Collagen-based hydrogels
Collagen is the most prevalent ECM protein, providing mechanical support to vertebrate connective tissues and promoting cell proliferation, migration, and differentiation [83,98,99]. The main structure of collagen is a triple-stranded helix stabilized by intra- and inter-chain hydrogen bonding, which is responsible for collagen’s thermo-responsive activity [100,101]. Due to its low antigenicity and high mechanical strength, collagen is often proposed for the construction of fibrous matrices in organoid culture [67,84,85,102]. In particular, the biomimetic properties of collagen make its hydrogel amenable to cell adhesion without modification and capable of presenting a native viscoelastic environment for resident cells [98].
Collagen-based hydrogels have been shown to facilitate the growth of mouse and human organoids for the gastrointestinal system, including the small intestine, colon, and stomach, in non-toxic, favorable environments for both organoids and normal tissue [86]. These collagen hydrogels exhibit thermo-responsive behavior, transforming into a solution at lower temperatures of 4 to 8 °C and a gel when heated to 37 °C. When compared to Matrigel-grown organoids, the organoids produced in collagen hydrogels had identical shapes, specific markers, and proliferation rates. Notably, in an ethylenediaminetetraacetic acid-colitis animal model, the transplantation of mouse colon organoids with the collagen hydrogel resulted in effective engraftment in injured tissue, showing the usefulness of regenerative medicine in vivo [86]. After in vivo engraftment, mouse and human intestinal organoids co-cultured with intestinal subepithelial myofibroblasts in collagen hydrogel recapitulated an autonomous experimental stem cell niche [85,87]. Furthermore, centimeter-long macroscopic units of intestinal epithelium were generated by embedding intestinal organoids in a floating collagen hydrogel [84]. Proliferating organoids aligned and fused to form a hollow structure of epithelial tubes containing all intestine-specific cell types, including Lgr5+ stem cells. The combination of collagen and CNFs has been investigated as a way to produce hybrid hydrogels with improved mechanical properties and bioactive effects, allowing the embedded crypts to undergo epithelial budding while maintaining cell viability and metabolic activity and expressing tissue-specific cell markers [88].
Human basal cells placed in floating collagen hydrogels grew mammary gland organoids, demonstrating the role of mechanical signals in controlling ductal branch elongation [89]. This process involved the cells migrating back and 4th within the surrounding collagen network, generating tension, promoting branch outgrowth, and causing plastic deformation of the matrix. The identified equilibrium of mechanical tension has been suggested as a promising area for future research into branching morphogenesis during organogenesis.
2. Gelatin-based hydrogels
Gelatin is a fibrous protein generated from the denaturation or hydrolysis of native collagen that is more water-soluble and less immunogenic than collagen [103–105]. It forms gels at approximately 30 °C by transitioning gelatin chains from disordered random coils to ordered helices through hydrogen bonding [106]. This process results in an intermediate biological complexity matrix following in situ gelation [101]. In addition, several advantageous properties of gelatin, such as biocompatibility, biodegradability, and the capability to promote cell adhesion and proliferation, make it highly attractive for organoid applications [103,105]. However, the poor mechanical properties and short degradation times, especially under physiological conditions, of native gelatin necessitate crosslinking for practical applications [107]. The covalent crosslinking of a gelatin-based hydrogel with 8-arm PEG was achieved through an enzymatic reaction with coagulation factor XIII (FXIII) [90]. This hybrid hydrogel demonstrated the ability to support cell differentiation and matrix secretion for liver organoid creation, with the potential to further stimulate tissue development by fine-tuning hydrogels and covalently immobilizing essential proteins.
3. Fibrin-based hydrogels
Fibrin is a blood protein formed by the activation of the serine protease thrombin during the coagulation process [108]. It possesses an abundance of naturally occurring RGD adhesion domains, which makes it useful as a substrate for cell proliferation and improved ECM deposition [108,109]. However, like other natural biopolymeric materials, fibrin has poor mechanical properties, necessitating a crosslinking approach [98]. FXIII-mediated enzymatic crosslinking of fibrin-based hydrogels has been used to enable the development of mouse intestinal organoids, as well as human intestinal, pancreatic, and liver organoids [48]. Notably, the combination of internal pressure and increased cell contractility inside the hydrogel enabled the formation of budding intestine organoids. Furthermore, laminin supplementation supported the long-term development of all tested epithelial organoids, demonstrating its effectiveness as a specific alternative to Matrigel.
An oil-free droplet microfluidic method was designed to fabricate hydrogel capsules with a fibrin hydrogel core and an alginate-chitosan composite shell in a single step (Fig. 4A) [91]. The produced hydrogel capsules with the prescribed compositions demonstrated good homogeneity and stability, as well as outstanding biocompatibility and high-throughput productivity. Human iPSC-derived hepatic cells self-organized into liver organoids with consistent sizes, composed of hepatocyte- and cholangiocyte-like cells in the core hydrogel generated by the enzymatic reaction of fibrinogen and thrombin (Fig. 4B). The produced liver organoids demonstrated the preservation of liver-specific activities, such as urea production and albumin secretion, indicating a successful recapitulation of the fundamental properties of the human liver (Fig. 4C and 4D).
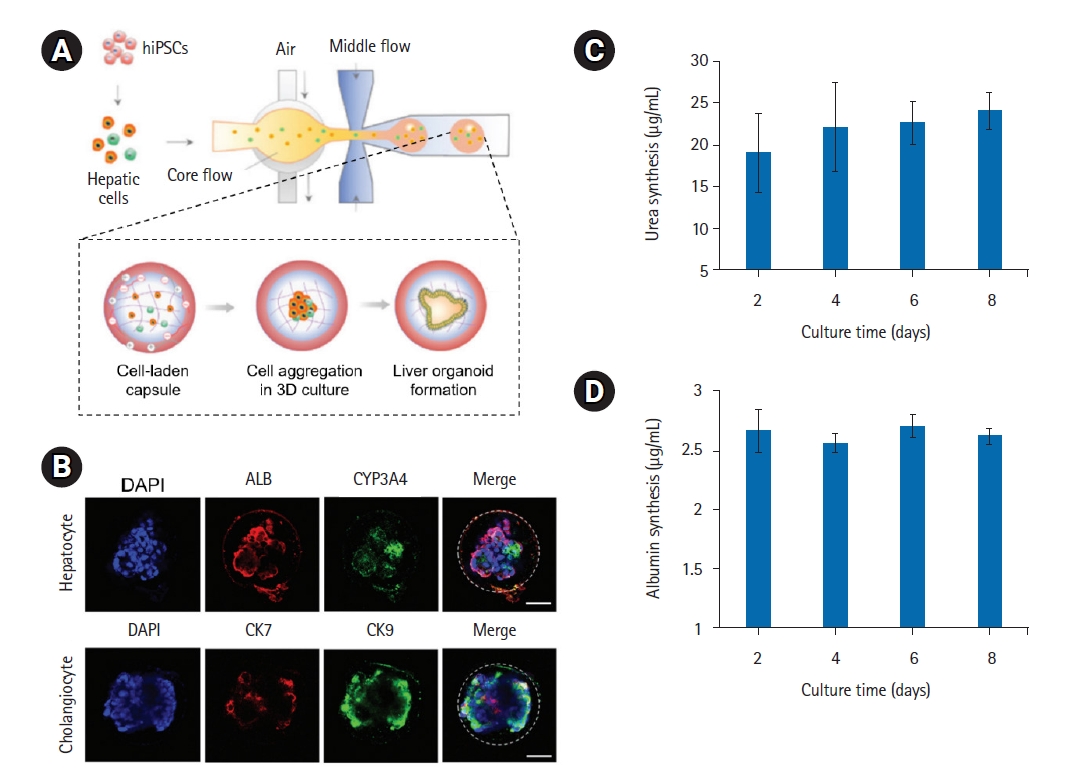
Fibrin-based composite hydrogel capsules for culture of liver organoids. (A) Schematic description of the oil-free droplet microfluidic system to fabricate composite hydrogel capsules with a fibrin hydrogel core and alginate-chitosan composite shell. (B) Immunohistochemical staining images of hepatocyte markers (ALB and CYP3A4) and cholangiocyte markers (CK7 and CK19) in liver organoids after 7 days of encapsulation in capsules. Scale bars: 50 μm. (C) Albumin secretion and (D) urea synthesis in liver organoids after encapsulation of 2, 4, 6 and 8 days. Reproduced from Wang et al. Biomater Sci 2020;8:5476–88, with permission from the Royal Society of Chemistry [91].
4. Recombinant protein-based hydrogels
Over the past two decades, recombinant protein-based hydrogels have advanced rapidly, thanks to considerable improvements in recombinant DNA technology and protein engineering [110–113]. In general, recombinant protein-based hydrogels demonstrate superior mechanical properties and consistency between batches when compared to their natural protein-based counterparts [110,114–116]. Genetic engineering of the amino acid sequence allows for precise control over the structural and functional aspects of protein building blocks, such as folding structure, chain length, and stereochemistry [117]. Specifically, the integration of signaling sequences into recombinant proteins provides the building blocks for artificial microenvironments that mimic the ECM [118–120].
Elastin-like proteins (ELPs) are recombinantly produced protein polymers consisting of conserved repeating units, as observed in tropoelastin’s hydrophobic domains (Fig. 5A) [121]. The pentapeptide Val-Pro-Gly-Val-Gly (VPGVG) is the most common repeating motif with lower critical solution temperature phase behavior [121,122]. Most ELPs are composed of the Val-Pro-Gly-X-Gly (VPGXG) pentapeptide repeat, where different physicochemical features may be accurately programmed depending on which amino acid is present in the guest residue “X” [123]. ELPs offer a modulable design due to their inherent biocompatibility, biodegradability, stimuli-responsiveness, and viscoelastic properties, making them potential biomaterials for 3D cell cultures, including ECM mimetics [123]. A genetic union of an ELP-based structural backbone with a fibronectin-derived, cell-adhesive, extended RGD sequence resulted in the development of a recombinantly designed ECM (Fig. 5B) [92]. Engineered ECM-based hydrogels were created by chemically crosslinking lysine residues with tetrakis(hydroxymethyl) phosphonium chloride and provided a microenvironment suitable for the formation and growth of mouse intestinal organoids by providing cell adhesive biochemical cues and elastomeric biomechanical cues. A recombinant triblock protein (PEP) made up of two leucine zippers (P) separated by an ELP (E) was also used to create a 3D matrix for organoid development (Fig. 5C) [93]. The PEP protein can self-associate into hydrogels thanks to its coiled-coil helix domains. Furthermore, combining PEP with cell-binding ECM motifs derived from fibronectin or laminin alpha 3, which are key components in pancreatic endocrine activities, resulted in the formation of pancreatic organoids composed of primary endocrine and endocrine progenitor cells.

Molecular structures of elastin-like proteins (ELPs). (A) Schematic representation of amino acid sequence domain arrangement in tropoelastin. Yellow rectangles indicate hydrophobic domains. Reproduced from Acosta et al. Adv Funct Mater 2020;30:1909050, with permission from John Wiley and Sons [123]. (B) Scheme of an engineered extracellular matrix (eECM) polypeptide chain with an extended, cell-adhesive RGD domain. Reproduced from DiMarco et al. Biomater Sci 2015;3:1376–85 [92]. (C) Scheme of PEP-FN and PEP-LAMA3 proteins containing leucine zipper (green), ELPs (yellow), and the cell binding peptide (blue). Reproduced from Kozlowski et al. Front Bioeng Biotechnol 2023;11:1144209, according to the Creative Commons license [93].
Spider silk, due to its exceptional biocompatibility and unique mechanical properties, can be utilized to construct 3D matrices for organoid culturing [124]. Recombinant spider silk, which is inspired by its natural counterpart, has been suggested as a consistent and highly reproducible scaffold material for 3D cell culturing [125]. Microfibers of recombinant spider silk protein undergo thermal transition polymerization, resulting in sturdy, elastic, and biocompatible matrices that facilitate the self-assembly of human PSCs into cerebral organoids [94,95]. When human PSCs are introduced into these silk microfiber networks, they stimulate neuroectoderm development and organoid maturation in relation to neuronal functioning [95].
5. Peptide-based hydrogels
Peptides, which are short chains of naturally occurring amino acids (usually 2 to 50 residues) connected by peptide bonds, can also be used to combine the beneficial qualities of natural and synthetic matrices for organoid cultures [126]. The simplicity of peptides’ structure allows for a more predictable design of hydrogels based on their sequences, compared to recombinant proteins, despite their relatively lower mechanical properties [127]. Among these, self-assembling peptides have been engineered to spontaneously form fibrillar structures in aqueous solutions, leading to physical gelation with architecture and characteristics similar to native ECM [127,128]. When human iPSCs were implanted in self-assembling peptide hydrogels, they successfully generated kidney organoids with complex architectures comparable to those in Matrigel [96]. Moreover, chemically crosslinked peptide hydrogels composed of specific ECM protein-mimicking fragments, such as collagen-like peptide (CLP) and CLP combined with RGD peptide (CLP-RGD), showed improved neural cell differentiation. This resulted in the rapid development of self-assembled cerebellar organoids [97]. Primary cerebellar cells spontaneously organized into tissue-like clusters capable of producing action potentials within the elastomechanical environment of ECM-mimetic matrices of CLP-based hydrogels.
Summary and perspectives
In this review, we highlight recent advances in natural hydrogels composed of polysaccharides or proteins, specifically for the production of organoids. These hydrogels have significant biological and biomedical applications, including developmental studies, disease modeling, drug screening, and regenerative medicine. Natural biopolymer-based hydrogels, due to their inherent benefits such as high activity (for instance, a wealth of cell recognition motifs), superior biocompatibility, and excellent degradability, have proven to be ideal for organoid culture. This is because they can mimic the microenvironmental features of the ECM. The molecular behaviors of natural biopolymers can be altered through physical or chemical crosslinking, chemical modification, or by combining them with other materials. This results in 3D matrices with mechanical and biochemical properties that encourage organoid growth, proliferation, or differentiation. The use of such natural biopolymeric hydrogels holds significant potential, especially for transplantation treatments. This is because they can reduce health risks associated with synthetic polymers, such as unwanted immunogenicity and in vivo toxicity [11].
However, significant material considerations need to be addressed for biopolymeric hydrogels to replace traditional Matrigel and be used as next-generation matrices in organoid technology: (1) Due to the lot-to-lot variability of biopolymers, consistently controlling the size and cellular composition of biopolymeric hydrogel-based organoids is a significant problem [129–134]. More comprehensive efforts to standardize the structural and functional features of biopolymeric hydrogels are required to make bioengineered matrices more effective and predictable. (2) To accurately replicate the dynamic process of organoid formation, the matrix material must be engineered to modify the microenvironment and surrounding stromal matrix in a spatiotemporally controlled manner. Natural biopolymers can change their physical and biochemical characteristics in response to stimuli commonly found in biological environments, such as light, temperature, and pH [10,135]. By controlling biomaterial parameters such as structural geometry, mechanical properties, and cell-binding ligands, this inherent stimulus-responsive behavior can be advantageously applied to the organoid system to better mimic the dynamic changes in extracellular microenvironmental inputs derived from the evolution of biological properties. (3) Integrative solutions combining biopolymeric hydrogel-based organoids with droplet microfluidics are highly desired as a future approach in organoid engineering. This approach would provide a programmable 3D scaffold for synthesizing organoids in a high-throughput manner. Recent studies suggest that microfluidic technologies, which allow for precise control of organoids and dynamic physical conditions, could be used to produce large quantities of highly uniform organoids in biopolymeric microdroplet hydrogels [136]. While this method holds great potential, especially for high-throughput drug candidate screening [137,138], it is still in the early stages of development. (4) Through 3D methods such as bioprinting, biopolymeric hydrogel-based organoids could be used as a building block to assemble larger, more complicated structures resembling genuine organs [139–142]. The design of biopolymeric hydrogels should allow for the embedding of organoids while incorporating their unique mechanical and biochemical properties to achieve the desired bulk properties. This would allow for the inclusion of key tissue compartments of native organs, such as the immune system and vascularization.
By addressing the aforementioned challenges through the integration of multiple disciplines, such as stem cell biology and materials engineering, natural biopolymer-based hydrogels could become an emerging framework to achieve cellular diversity, maturation, and full functionality of organoids with greater controllability and fidelity, facilitating an eventual shift away from the use of Matrigel and opening up a large design space to transform the field of numerous downstream translational applications.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (grant number: 23A0105L1) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Korea (grant number: HI22C1754).
Data availability
Please contact the corresponding author for data availability.




