Mimicking microbone tissue by 3-dimensional printing
Article information
Abstract
Bones are mineralized connective tissues composed of osteoclasts, osteoblasts, and osteocytes. While bone is one of the few tissues that can regenerate in adulthood, its regeneration is limited in the case of large bone defects due to an environment that is detrimental to bone formation, which can be caused by soft tissue injury and impeded vascularization, ultimately reducing the potential for significant bone creation. Consequently, recent research has focused on tissue engineering and regenerative medicine to address these complex issues. This article reviews recent major advances in the cell components used for bone regeneration studies, specific markers of bone differentiation, 3-dimensional (3D) printing techniques for the structural mimicry of bones, and the use of natural and synthetic biomaterials. Functional bone structures and bone organoids can be created using 3D printing, which allows the reconstruction of bone tissue by attaching living cells to scaffolds. These scaffolds are designed with appropriate shapes and mechanical properties to mimic the bone microenvironment. The application of 3D printing in the development of bone organoids holds promise for providing improved solutions for the development of test systems for disease modeling and drug development.
Introduction
Organoids are defined as 3-dimensional (3D) structured in vitro biological complexes that contain one or more cell types and that partially replicate the form and function of their in vivo counterparts [1]. Organoids derived from adult stem cells take advantage of the cell-driven tissue regeneration process and can be generated directly from the healthy or damaged epithelium of various organs [2]. Although bones are resilient tissues with self-healing properties, they cannot repair large bone defects on their own [3]. In such cases, surgical interventions such as bone grafts are necessary to restore bone function [4]. Bone is a rigid yet highly dynamic tissue that provides support and protection for the body’s organs. It consists of living bone cells organized within a biomineral matrix. The matrix’s intertwined cell layers are reinforced to form bone, with collagen fibers and inorganic bone minerals, present as small crystals, comprising the majority of the bone structure [5,6]. Therefore, scaffolds, cells, and cytokines are crucial for bone repair. Scaffolds are 3D structures that offer a temporary matrix for extracellular matrix formation and cellular activity, facilitating oxygen diffusion, nutrient delivery, and waste removal [7]. However, traditional scaffold fabrication methods often lack precise control over the scaffold's architecture, including chemical composition, porosity, pore size, shape, and network, leading to suboptimal 3D bone scaffolds [8]. In contrast, 3D printing and additive manufacturing technologies are emerging methods capable of creating customized scaffolds with precise control over both internal and external structures, using a variety of materials such as metal, ceramic, and polymer [9]. This review summarizes the structures and cell types of human bone tissue and bone models that mimic in vivo conditions. It specifically focuses on recent manufacturing methods, including 3D printing, and the future applications of bone organoids, which hold potential for investigating bone diseases and testing promising therapeutic drugs.
Mechanism of bone formation
Ethics statement: This study was a literature review of previously published studies and was therefore exempt from institutional review board approval.
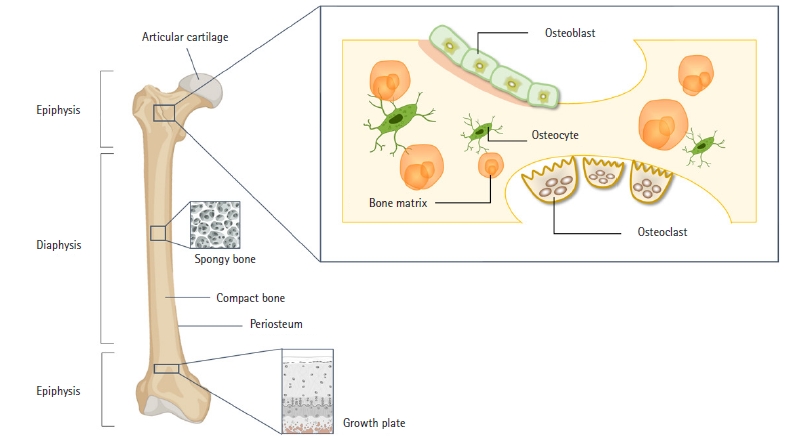
Bones perform many functions, including serving as the main structural framework to which muscles, ligaments, and tendons attach, providing mechanical support, and safeguarding vital organs. Additionally, bone marrow contributes essential minerals and facilitates hematopoiesis [5,10]. Bones consist of osteoblasts, osteoclasts, and osteocytes, and a balanced interplay between osteoblasts and osteoclasts is crucial for maintaining normal bone homeostasis (Fig. 1) [11]. Bone regeneration is a complex series of biological processes that involve a range of cell types and both intracellular and extracellular molecular signaling pathways. These processes follow a specific temporal and spatial sequence aimed at optimizing skeletal repair and restoring function [12]. Several biomarkers for bone formation and resorption have been identified, with changes in their expression primarily associated with the bone remodeling process, which is linked to bone and cartilage degradation as well as various pathological conditions (Fig. 2).
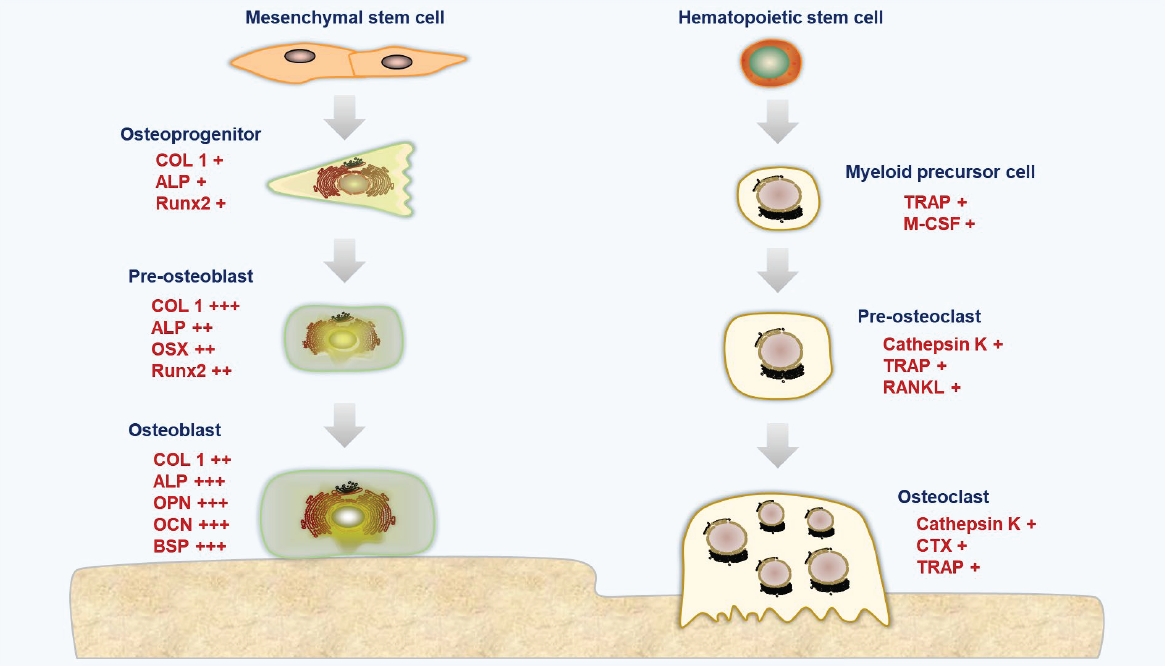
The regulation of bone remodeling by cellular molecules. COL1, collagen type Ⅰ alpha 1; ALP, alkaline phosphatase; RUNX2, RUNX family transcription factor 2; OSX, osterix; OPN, osteopontin; OCN, osteocalcin, BSP, bone sialoprotein; TRAP, tartrate-resistant acid phosphatase; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factors kB ligand; CTX, C-terminal lopeptide. Illustrated by the authors.
The key cells responsible for bone formation are osteoblasts. The specification of osteoblasts for the skeletal lineage can be divided into 3 distinct stages: osteoprogenitors, proosteoblasts, and osteoblasts with increasing differentiation [11,13]. Type I collagen (COL I) constitutes 90% of the collagen found in bone tissue and is responsible for forming the triple helices of polypeptides that comprise collagen fibrils. These fibrils then assemble into higher-order structures, such as fibril bundles and fibers, through interactions with other collagenous and noncollagenous proteins [14]. Alkaline phosphatase (ALP) is highly expressed in cells of mineralized tissues and plays a crucial role in the development of hard tissues. ALP facilitates mineralization by increasing the local concentration of inorganic phosphate and reducing the level of extracellular pyrophosphate, which is an inhibitor of mineral formation [15]. Runt-related transcription factor 2 (RUNX2) is a pivotal transcription factor that governs the development of osteoblasts. The expression of RUNX2 in bone and at the osteogenic front of a suture is essential for the closure of cranial sutures and the morphogenesis of membranous bone. Consequently, RUNX2 is tightly regulated by a series of posttranslational modifications through the sequential recruitment of various enzymes [16]. Osterix, a member of the SP/KLF family, stimulates the expression of several genes associated with mature osteoblasts, including COL I, osteonectin, osteopontin (OPN), osteocalcin (OCN), and bone sialoprotein (BSP). These genes are vital for the activity of osteoblasts during the ossification process [17,18]. OPN, a noncollagenous extracellular glycoprotein found in bone, is considered a critical component in the attachment of osteoclasts to the bone matrix during the resorption process [19]. OCN, the most abundant noncollagenous protein in the bone extracellular matrix, is exclusively synthesized by osteoblasts and can be detected in the bloodstream following bone resorption [20]. Carboxylated, undercarboxylated, and uncarboxylated OCN are the 3 primary types of OCN. Vitamin K is required for the carboxylation process, which increases OCN's affinity for the calcium ions in hydroxyapatite (HA) [21]. BSP is a strong matrix-associated signal that promotes osteoblast development and enhanced formation of a mineralized matrix, in addition to being an excellent mineralization nucleator [22].
Osteoclasts facilitate bone loss in pathological situations by increasing their resorptive activity. These cells originate from osteoclast precursors within the bone marrow’s monocyte/macrophage lineage, which in turn are derived from hematopoietic stem cells [23]. The development and activity of osteoclasts are regulated by various cytokines, particularly osteoprotegerin (OPG), a natural inhibitor of the receptor activator of NF-κB ligand (RANKL). OPG binds to RANKL, preventing its interaction with the receptor activator of NF-κB (RANK) [24]. Activation of the RANKL/RANK signaling pathway triggers the expression of several genes that govern osteoclast differentiation and function, including cathepsin K, tartrate-resistant acid phosphatase (TRAP), and C-terminal telopeptide of type I collagen (CTX). The presence of osteoclast and macrophage activity can be detected by measuring the activity of tartrate-resistant acid phosphatase type 5 (TRAP or Acp5), a glycoprotein secreted by mature osteoclasts, active dendritic cells, and macrophages [25]. The proliferation and differentiation of osteoclasts largely depend on the hematopoietic growth factor macrophage colony-stimulating factor [26]. Cathepsin K, one of the most potent proteases in the lysosomal cysteine protease family, plays a crucial role in degrading COL I and elastin during bone resorption [27]. Osteoclasts produce cathepsin K, which is not only a marker of bone resorption but also reflects osteoblast activity, as it breaks down and releases CTX from type I collagen [28].
Application of 3D printing technologies to bone repair and regeneration
1. Manufacturing using several 3D printing technologies
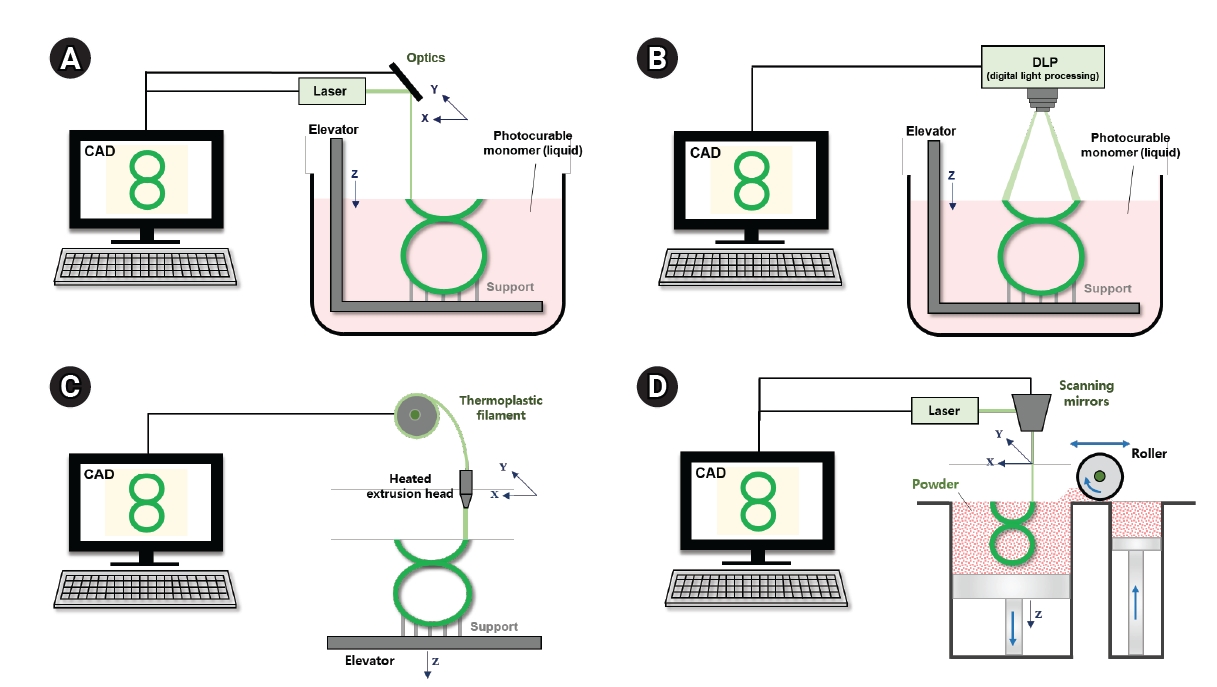
The purpose of tissue engineering scaffolds is to produce customized scaffolds with pre-engineered shapes, structures, and functionalities for improved tissue regeneration [29]. Because 3D printing yields structures that morphologically resemble the multiscale architecture of human tissue, it has been recognized as a useful method for creating tissue engineering scaffolds. Bone tissue engineering, utilizing 3D printed scaffolds, has become a promising approach for bone reconstruction and treatment, overcoming the limitations of traditional bone grafts [3]. Unlike subtractive manufacturing techniques, 3D printing, also known as additive manufacturing, solid freeform fabrication, or rapid prototyping, builds objects from 3D model data, usually by adding material layer by layer [29]. The most commonly used 3D printing techniques that employ solid polymers for product creation include fused deposition modeling (FDM), stereolithography (SLA), digital light processing (DLP), and selective laser sintering (SLS) (Fig. 3). FDM, one of the earliest 3D printing methods, fabricates 3D scaffolds using a thermoplastic polymer filament, which is extruded through a heated nozzle onto a platform where it hardens and sets [30]. Biocompatible and biodegradable polymers such as polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL) are frequently used for implants. Additionally, many pharmaceutical materials, including cellulose derivatives, polyvinyl alcohol, Eudragit, ethylene vinyl acetate, polyethylene oxide, and thermoplastic polyurethane have been successfully applied in FDM [31]. Laser-based 3D printing systems use the energy of a laser beam or ultraviolet (UV) light to fuse materials. Charles Hull invented the first 3D printing method, SLA, and filed a patent for it in the mid-1980s. SLA, a widely used technology based on vat photopolymerization, selectively polymerizes exposed photocurable resins through various chemical reactions when exposed to light [32]. However, the harshness of UV-based crosslinking, extensive post-processing, inadequate mechanical properties, the potential for liquid resin to become trapped within the finished product, and the lack of biocompatible and biodegradable materials have limited its biomedical applications [33]. The DLP technique, which uses a mask projection method instead of SLA’s approach, offers the advantage of faster production times, essential for treating severe bone injuries. DLP is a light-based technology that accurately produces 3D structures, facilitating the simulation of complex internal bone topologies akin to porous cancellous bone [34]. However, DLP also relies on photocuring, sharing the same drawbacks as SLA, including the harshness of UV-based crosslinking, extensive post-processing, inadequate mechanical properties, and a shortage of suitable biocompatible or biodegradable materials. In the SLS process, a focused laser selectively scans polymer powder material just below its melting point [35]. The primary advantage of SLS for bone tissue engineering is the 3D printing of metals like titanium and ceramics like HA. However, the downsides include the energy consumption and high costs associated with the sintering phase of SLS manufacturing equipment [36]. Moreover, increasing the laser's energy can enhance mechanical strength by melting the material rather than just sintering it.
2. Materials used in bone tissue engineering
Bone organoids constructed from bioactive materials are 3D self-regenerating and self-organizing microbone tissues with biomimetic spatial characteristics [37,38]. In order to stimulate bone regeneration, porous bone tissue-engineered scaffolds with the right shape, pore size, porosity, degradability, biocompatibility, mechanical properties, and cellular responses are needed [29]. A material with outstanding biological and mechanical properties is required for use in 3D printing to fabricate scaffolds for bone tissue regeneration. Various types of materials are employed in scaffold construction, including synthetic materials, natural materials, ceramics, and composite materials (Table 1) [39–50]. Due to the limitations of HA and calcium sulfate as bioceramic materials, β-tricalcium phosphate (β-TCP) has become the most widely used [51]. TCP is a bone replacement biomaterial with the chemical formula Ca3(PO4)2, which may be converted at certain temperatures into 2 different α-TCP or β-TCP phases. The crystallinity and compressive strength of α-TCP are stronger than those of β-TCP due to its higher sintering temperature; however, its biological activity is ineffective compared to β-TCP. In addition to having good osteoconductivity and biocompatibility, β-TCP degrades in the body and is 10 to 15 times more soluble than HA under typical conditions [52]. Fairag et al. [40] reported that scaffolds made of pure polymer (poly[lactic acid], 100 M, Lactoprene; Poly-Med Inc., Anderson, SC, USA) and β-TCP, in particular, demonstrated exceptional mechanical properties, biological characterization, and supported bone matrix formation. However, β-TCP has disadvantages such as slow disintegration and an inability to prevent bone resorption [53]. Incorporating trace elements is crucial for bone bonding in the human body. Consequently, a significant area of potential research involves integrating trace metals such as strontium, magnesium, zinc, silver, and silicon into scaffolds to enhance bone tissue regeneration. Liu et al. [41] found that strontium-containing hydroxyapatite (SrHA) could increase osteoblast activity, promote proliferation, and enhance ALP activity. This suggests that 3D printing technology can efficiently produce PCL/SrHA composite scaffolds, which show great potential as implantable materials for bone tissue engineering applications. Additionally, it was observed that a micro/nano-HAp surface layer promoted mouse bone mesenchymal stem cell adhesion, osteogenic differentiation, and the expression of angiogenic genes in human umbilical vein endothelial cells [42]. Zinc (Zn) and its alloys are promising biomaterials with favorable mechanical strength, degradation rates, and biocompatibility [54]. The authors noted that the role of Zn in osteogenesis and osteoclastogenesis is associated with the Wnt/β-catenin and NF-κB signaling pathways, respectively. They also indicated that PCL/Zn scaffolds progressively enhanced osteoclastogenesis with increasing Zn concentration [43].
Polymers are commonly used as synthetic materials for creating scaffolds, with linear aliphatic polyesters such as PGA, PLA, and PLGA being the most widely used [39]. Additionally, biocompatible polymers like polyether ether ketone, PCL, and PLA are utilized in tissue engineering to replace bone tissue due to their biodegradable, non-toxic, non-immunogenic, and non-inflammatory properties [55]. Ledda et al. [50] proposed the use of 3D-printed PLA scaffolds with a microstructure inspired by trabecular bone architecture to support the adhesion, growth, and differentiation of osteoblast-like cells and microporous structures. They demonstrated that these scaffolds mimic natural bone hierarchies and promote increased bioactivity. Moreover, Zhang et al. [44] developed a scaffold that imitates Haversian bone, designed as a multicellular delivery system with osteogenic and angiogenic/neurogenic cells distributed in specific locations. This system actively supports bone tissue engineering and serves as a proof-of-concept for a 3D structure-based co-culture platform. Due to its biocompatibility, biodegradability, and adaptability, PCL, a thermoplastic polymer, is regarded as a polymer material appropriate for the fabrication of porous scaffolds utilized in bone tissue engineering [56]. Rosales-Ibáñez et al. [45] found that grafting gelatin molecules onto PCL, which is inherently hydrophobic and inhibits cell attachment, enhances cell adhesion, and increases cell survival. Ebrahimi et al. [46] reported that coating PCL scaffolds with HA/collagen (COL) creates a bone-like surface that accelerates healing and that surface roughness enhances cell adhesion and proliferation. In our previous study, we used 3D printing and PCL to fabricate a scaffold loaded with rifampicin and cefazolin for osteomyelitis treatment. By employing computer-aided design and manufacturing (CAD/CAM) and printing rifampicin-loaded PCL scaffolds at 80°C, we successfully preserved the antibacterial activity of both drugs [57,58]. Bioactive glass (BG) is a well-known osteoinductive and osteoconductive material that has been combined with biopolymers to create composite scaffolds for bone engineering [59,60]. Distler et al. [49] developed PLA-BG composite filaments for 3D scaffold manufacturing and demonstrated the printability of these filaments, as well as the bioactivity and cytocompatibility of the resulting PLA-BG scaffolds, using pre-osteoblast MC3T3E1 cells. Luo et al. [47] described a novel biodegradable organic-inorganic composite scaffold with tailored porosity, mechanical strength, and excellent bioactivity as a potential candidate for bone regeneration. Various bioink materials have been explored for producing bone-like scaffold additives to demonstrate bone conductivity. However, clinical applications have been only partially successful due to issues such as inadequate bone conduction signals, biomaterial-related infections, suboptimal cell survival, lack of reproducibility, and insufficient patient specificity [48].
An osteoregenerative bioink capable of being printed into complex geometries at high production rates is known as hyperelastic bone (HB) [61]. Due to its distinct biomechanical and biochemical properties, HB bioink can facilitate new bone growth and vascularization without requiring additional biological components [48]. Researchers have demonstrated the ability to 3D-bioprint highly porous, reproducible, and defect-specific bone grafts using HB bioink.
3D printing approaches to bone organoids for bone regeneration
The creation of bone organoid structures is challenging due to the dense, mineralized nature of bone and the difficulty in accessing the cellular components within. Therefore, the functionality of a bone organoid can be achieved by cultivating and organizing various cells on a scaffold. When selecting scaffolds for bone organoids, factors such as biodegradation rate, mechanical properties, structural resemblance to bone tissue, and biocompatibility with bone cells must be taken into account. By judiciously combining 3D printing with other fabrication techniques, it is possible to create bone tissue engineering scaffolds that possess intricate configurations and structures, thereby better replicating the architecture of natural bone tissue and enhancing bone regeneration. Although current techniques enable the fabrication of tissue structures, including bone tissue, the reconstruction of fully functional tissues remains a challenge. Research is ongoing to print live cells and incorporate growth factors and drugs; however, the post-printing viability of cells, as well as the stability of growth factors and drugs, must still be addressed. This approach necessitates thorough process characterization and further investigation [62,63].
Various studies have been conducted to develop bone organoids in vitro. Akiva et al. [64] created woven bone organoids using silk from silkworm cocoons and particulate leaching techniques, while Giger et al. [65] developed bone marrow organoids by aggregating endothelial and mesenchymal cells in a hydrogel microwell, aimed at drug screening and patient-specific analysis. In addition, Nilsson produced scalable micro-organoids from human periosteum-derived cells and differentiated them into callus organoids for use in mouse long bone reconstruction [66]. Furthermore, Hall investigated cartilage organoids with a hierarchical structure related to osteochondral tissue [67]. More recently, Park et al. [68] explored a trabecular bone organoid model for studying the control of localized bone reconstruction.
Studying normal bone organoids using 3D printing presents challenges, so this technology is being applied to create accessible osteosarcoma models and facilitate research. Contessi Negrini et al. [69] demonstrated that seeding human mesenchymal stem cells (hMSCs) on polyurethane scaffolds and inducing their osteogenic differentiation, followed by culturing osteosarcoma cells on the resulting biomimetic 3D printed scaffold after removing the hMSCs through lysis, can artificially replicate a chemically biomimetic microenvironment in vitro. Pan et al. [70] proposed that integrating 2D titanium carbide MXene with 3D-printed composite BG scaffolds could serve as a promising biomaterial system. This system has the potential to significantly accelerate tumor bone tissue growth in vivo and to initiate related tissue engineering studies. Zhang et al. [71] used 3D printing technology to create a Mg-PCL scaffold and test its effects and molecular mechanisms for preventing OS growth and metastasis. The results showed that the Mg-PCL scaffold might enhance bone formation and could be a good choice for bone tissue engineering. Recently, Zhang et al. [72] demonstrated that bone organoids can be produced using 3D bioprinting. In their study, they created functional osteocyte bone organoids by subjecting 3D-bioprinted hMSCs-laden graphene oxide composite constructs to long-term compressive stress for 8 weeks. In the test group that received mechanical stimulation from the first day, there were improvements in collagen I maturation, osteocyte differentiation, lacuna-canalicular network formation, and Yes-associated protein expression. However, the complexity of bone metabolism means that current research on bone regeneration and restoration, which aims to replicate the actual physiological microenvironment, is still lacking. Further studies are essential to enhance our understanding and optimization of bone structure imitation and intracellular molecular signaling networks for clinical applications.
Conclusion
In this study, we provide a summary of the structures and cell types that comprise human bone tissue, as well as in vivo models that mimic bone. We place a special emphasis on recent manufacturing methods, including 3D printing technologies. Furthermore, we discuss the application of 3D printing techniques in the creation of bone organoids for the purpose of bone regeneration. A significant amount of research is focused on reconstructing functional bone structures. This involves the use of osteosarcoma-derived scaffolds and bone organoids. By employing structures that possess the appropriate shapes and mechanical properties in both in vitro and in vivo studies, researchers can create a biomimetic microenvironment conducive to the reconstruction of bone tissue.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was funded by a grant from the National Research Foundation of Korea (NRF) grant (2020R1A2C200652811) and Korea Environment Industry & Technology Institute (KEITI) through -Core Technology Development Project for Environmental Diseases Prevention and Management Program, funded by Korea Ministry of Environment (MOE) (2021003310006).
Data availability
Please contact the corresponding author for data availability.
Acknowledgements
The authors thank MID (Medical Illustration & Design) for providing excellent support with medical illustration.