|
 |
| Organoid > Volume 1; 2021 > Article |
|
Abstract
NOTES
Funding
This study was supported by grants from the National Research Foundation funded by the Ministry of Science and ICT of Korea (No. 2019M3A9H1103718).
Fig. 1.
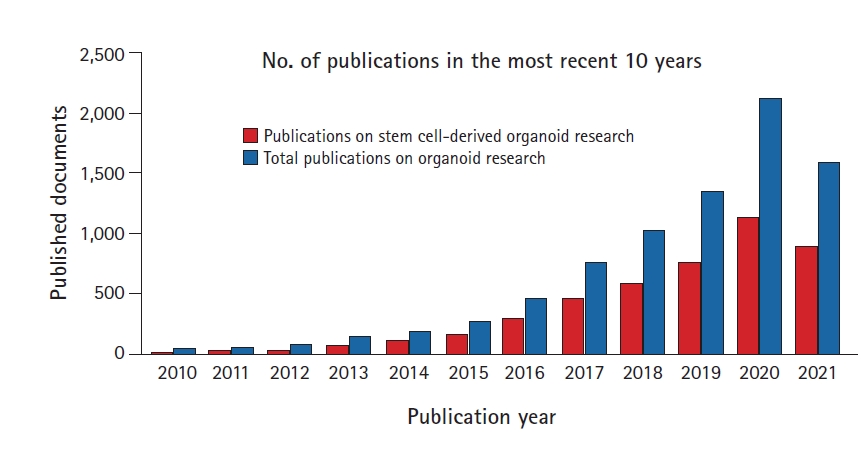
Fig. 2.

Fig. 3.

Fig. 4.
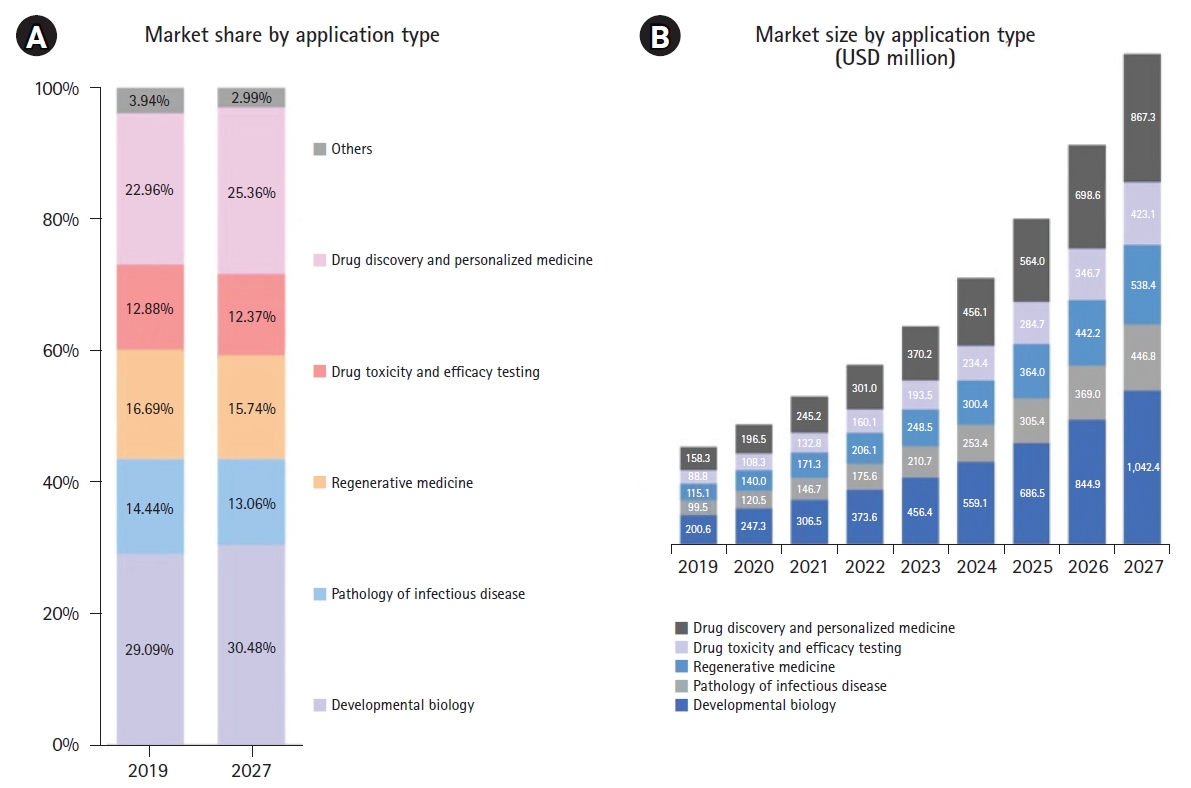
Fig. 5.
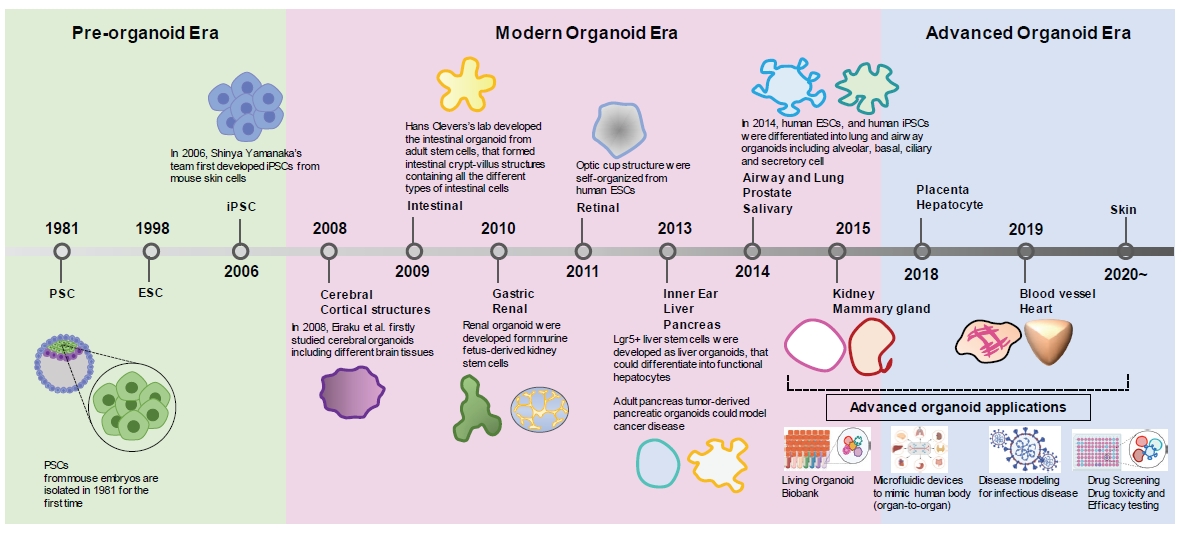
Table 1.
| Type | Cell type | Source | Key feature | Application | Ref |
|---|---|---|---|---|---|
| Ectoderm | Optic cup, vesicle and retina organoid | hESCs | · Formation of optic cup | · Developmental biology | [33,41] |
| hiPSCs | · Formation of rod and cone cells | · Disease modeling | |||
| · RPE-to-NR-transition | · Biobanks for academic studies | ||||
| Brain organoid and forebrain spheroids (brain assembloid) | hESCs | · Formation of cerebral cortical neurons, forebrain, hippocampus, radial glial cells | · Developmental biology | [35-37] | |
| hiPSCs | · Modeling human microcephaly, Timothy syndrome, and ZIKV infection | · Disease modeling | |||
| · Drug screening | |||||
| Skin organoid | hESCs | · Formation of epidermis and dermis layer capable of developing new hair follicle | · Developmental biology | [42] | |
| hiPSCs | · Formation of nascent mechanosensitive tough complexes of sensory neurons and Schwann cells that target Merkel cells | · Regenerative medicine | |||
| · Omics profiling | |||||
| Inner ear organoid | hESCs | · Formation of sensory epithelia | · Developmental biology | [20] | |
| · Formation of vestibular-like hair cells with electrophysiological properties similar to those of native sensory hair cells | |||||
| Surface ectoderm | Salivary gland | hASCs | · Developed ductal structures and mucin-expressing acinar-like cells | · Developmental biology | [21] |
| · Formation of salivary glands in vivo within the mouse embryonic salivary gland mesenchyme | · Regenerative medicine | ||||
| Mesoderm | Kidney organoid | hESCs | · Formation of metanephric and epithelial nephron progenitors | · Developmental biology | [22-24] |
| hiPSCs | · Use of human kidney organoids for the evaluation of nephrotoxicity | · Omics profiling | |||
| · Modeling of polycystic kidney disease | · Drug toxicity | ||||
| Cardiac organoid | hESCs | · Confirmation of regenerative capacity of immature human heart tissue | · Developmental biology | [25-27] | |
| hiPSCs | · Formation of cardiac organoid similar to human fetal/neonatal heart tissue | · Disease modeling | |||
| · Modeling regenerative response following cryoinjury | · Drug screening | ||||
| · Modeling of myocardial infarction and genetic cardiomyopathy | · Omics profiling | ||||
| Blood vessel organoid | hESCs | · Exhibition of morphological, functional and molecular characteristic of human microvasculature | · Transplantation | [28,29] | |
| hiPSCs | · DLL4 and NOTCH3 as key drivers of diabetic vasculopathy in blood vessels | · Developmental biology | |||
| · Vascularized organoids on a chip | |||||
| Endoderm | Lung organoid | hESCs | · Formation of engraftable alveolar and airway-like structures through basal, ciliary, and secretory cells | · Developmental biology | [30,31,38] |
| hiPSCs, hASCs | · Modeling of cystic fibrosis | · Regenerative medicine | |||
| · Modeling of SARS-CoV-2 infection | · Drug screening | ||||
| · Omics profiling | |||||
| Liver organoid | hiPSCs, hASCs | · Formation of a more complete 3D hiPSC-LB organoid by HUVEC and mesenchymal cell co-culture | · Regenerative medicine | [43] | |
| · Modeling of SARS-CoV-2 infection | · Disease modeling | ||||
| · Modeling of α1-antitrypsin deficiency and Alagille syndrome | · Omics profiling | ||||
| Gastric organoid | hESCs | · Modeling of human gastric cancer and Helicobacter pylori | · Developmental biology | [34] | |
| hiPSCs human primary cells | · Differentiation of gastric organoids into mucous and endocrine cell lineages | · Disease modeling | |||
| · Omics profiling | |||||
| Pancreatic organoid | hESCs human primary cells | · Formation of ductal and acinar cells similar to human fetal pancreas | · Developmental biology | [44] | |
| · Modeling of ductal pancreatic cancer | · Disease modeling | ||||
| · Omics profiling | |||||
| Intestinal organoid | hESCs | · Formation of characteristic villus and crypt-like structure | · Developmental biology | [39,40,45] | |
| hiPSCs human primary cells | · Modeling of congenital loss of intestinal enteroendocrine cells | · Regenerative medicine | |||
| · Production of CRISPR-Cas9-mediated CFTR-repaired intestinal organoid | · Disease modeling | ||||
| · Hydrogel-delivered human intestinal organoids could heal colon wounds | |||||
| Colon organoid | hESCs | · Modeling of colorectal cancer | · Developmental biology | [32] | |
| hiPSCs, hASCs human primary cells | · Mimicking of colonic crypts | · Regenerative medicine | |||
| · Contributes to colonic epithelium regeneration | · Disease modeling | ||||
| Prostate organoid | hASCs | · Formation of prostate organoid exhibiting functional androgen receptor signaling | · Developmental biology | [46] | |
| · Formation of architecture containing luminal and basal cell | · Disease modeling | ||||
| · Modeling of prostate cancer |
hESCs, human embryonic stem cells; hiPSCs, human induced pluripotent stem cells; RPE, retinal pigment epithelium; NR, neural retina; ZIKV, Zika virus; hASCs, human adult stem cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; 3D, three-dimensional; LB, liver bud; HUVEC, human umbilical vein endothelial cells.
Table 2.
| Type | Organoid | Cell source | Pathogen | Ref |
|---|---|---|---|---|
| Virus | Brain | PSCs | · Zika virus | [60] |
| · Japanese encephalitis virus | ||||
| · SARS-CoV-2 | ||||
| Liver | PSCs | · Hepatitis B virus | [61] | |
| ASCs | ||||
| Intestine | PSCs | · Human norovirus | [53,54] | |
| ASCs | · Rotavirus | |||
| · SARS-CoV-2 | ||||
| Kidney | ASCs | · BK virus | [57,62] | |
| · SARS-CoV-2 | ||||
| Respiratory tract | PSCs | · Respiratory syncytial virus | [31] | |
| ASCs | · Influenza virus | |||
| · Enterovirus 71 | ||||
| · SARS-CoV-2 | ||||
| Bacterium | Intestine | PSCs | · Salmonella typhi | [55,56] |
| ASCs | · Clostridium difficile | |||
| Stomach | PSCs | · Helicobacter pylori | [12] | |
| ASCs | ||||
| Respiratory tract | ASCs | · Clostridium difficile | [56] | |
| Parasite | Liver | PSCs | · Plasmodium | [63] |
| ASCs | ||||
| Intestine | PSCs | · Cryptosporidium | [56] | |
| ASCs | ||||
| Respiratory tract | PSCs | · Cryptosporidium | [56] | |
| ASCs |
Table 3.
References
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 8,394 View
- 266 Download
- ORCID iDs
-
Hanbyeol Lee

https://orcid.org/0000-0001-9671-315XJeong Suk Im

https://orcid.org/0000-0002-5292-2803Da Bin Choi

https://orcid.org/0000-0002-1162-8982Dong-Hun Woo

https://orcid.org/0000-0001-7470-018X - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print