2. Vacanti J, Vacanti CA. Chapter 1. The history and scope of tissue engineering. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. Amsterdam: Elsevier; 2014. p. 3-8.
4. Nerem RM, Schutte SC. Chapter 2. The challenge of imitating nature. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. 4th ed. Amsterdam: Elsevier; 2014. p. 9-24.
7. Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 2017;23:393-410.


11. Kucukgul C, Ozler SB, Inci I, Karakas E, Irmak S, Gozuacik D, et al. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol Bioeng 2015;112:811-21.


12. Jang J, Park HJ, Kim SW, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017;112:264-74.


19. Lavrentieva A. Essentials in cell culture. In: Kasper C, Charwat V, Lavrentieva A, editors. Cell culture technology. Cham: Springer; 2018. p. 23-48.
20. Bhatia SN, Balis UJ, Yarmush ML, Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog 1998;14:378-87.


21. Shimaoka S, Nakamura T, Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res 1987;172:228-42.


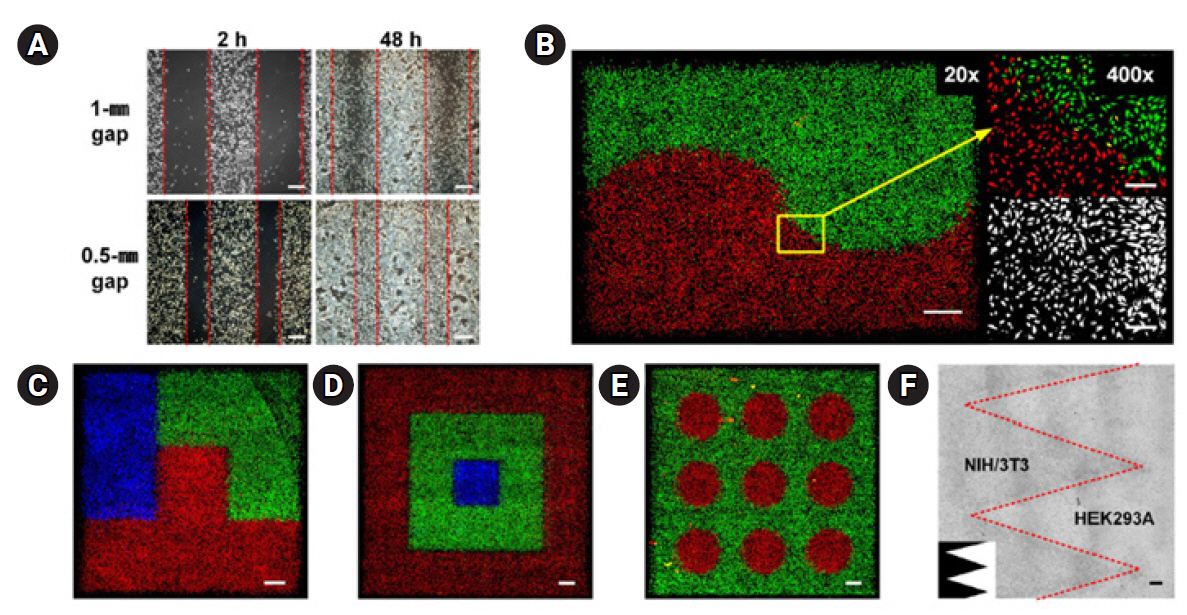
23. Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J Biomed Mater Res 1997;34:189-99.


25. Karp JM, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane DS, et al. A photolithographic method to create cellular micropatterns. Biomaterials 2006;27:4755-64.


26. Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials 1999;20:2363-76.


29. Zhang S, Yan L, Altman M, Lässle M, Nugent H, Frankel F, et al. Biological surface engineering: a simple system for cell pattern formation. Biomaterials 1999;20:1213-20.


31. Li Y, Yuan B, Ji H, Han D, Chen S, Tian F, et al. A method for patterning multiple types of cells by using electrochemical desorption of self-assembled monolayers within microfluidic channels. Angew Chem Int Ed Engl 2007;46:1094-6.


32. Tenstad E, Tourovskaia A, Folch A, Myklebost O, Rian E. Extensive adipogenic and osteogenic differentiation of patterned human mesenchymal stem cells in a microfluidic device. Lab Chip 2010;10:1401-9.

34. Chen CY, Barron JA, Ringeisen BR. Cell patterning without chemical surface modification: cell-cell interactions between printed bovine aortic endothelial cells (BAEC) on a homogeneous cell-adherent hydrogel. Appl Surf Sci 2006;252:8641-5.

35. Kim JD, Choi JS, Kim BS, Choi YC, Cho YW. Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer 2010;51:2147-54.

37. Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;26:3124-30.


39. Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, et al. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 2012;18:33-44.


41. Sanjana NE, Fuller SB. A fast flexible ink-jet printing method for patterning dissociated neurons in culture. J Neurosci Methods 2004;136:151-63.


42. Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, In Het Panhuis M. Bio-ink for on-demand printing of living cells. Biomater Sci 2013;1:224-30.


43. Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013;34:130-9.


44. Yanez M, Rincon J, Dones A, De Maria C, Gonzales R, Boland T. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng Part A 2015;21:224-33.


45. Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004;25:3707-15.


46. Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials 2005;26:93-9.


47. Millet LJ, Collens MB, Perry GL, Bashir R. Pattern analysis and spatial distribution of neurons in culture. Integr Biol (Camb) 2011;3:1167-78.


48. Arai K, Iwanaga S, Toda H, Genci C, Nishiyama Y, Nakamura M. Three-dimensional inkjet biofabrication based on designed images. Biofabrication 2011;3:034113.


49. Gruene M, Pflaum M, Deiwick A, Koch L, Schlie S, Unger C, et al. Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 2011;3:015005.


50. Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 2010;6:2494-500.


51. Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010;31:7250-6.


52. Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, et al. Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods 2010;16:847-54.


53. Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013;25:5011-28.


54. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016;76:321-43.


55. Hewes S, Wong AD, Searson PC. Bioprinting microvessels using an inkjet printer. Bioprinting 2017;7:14-8.

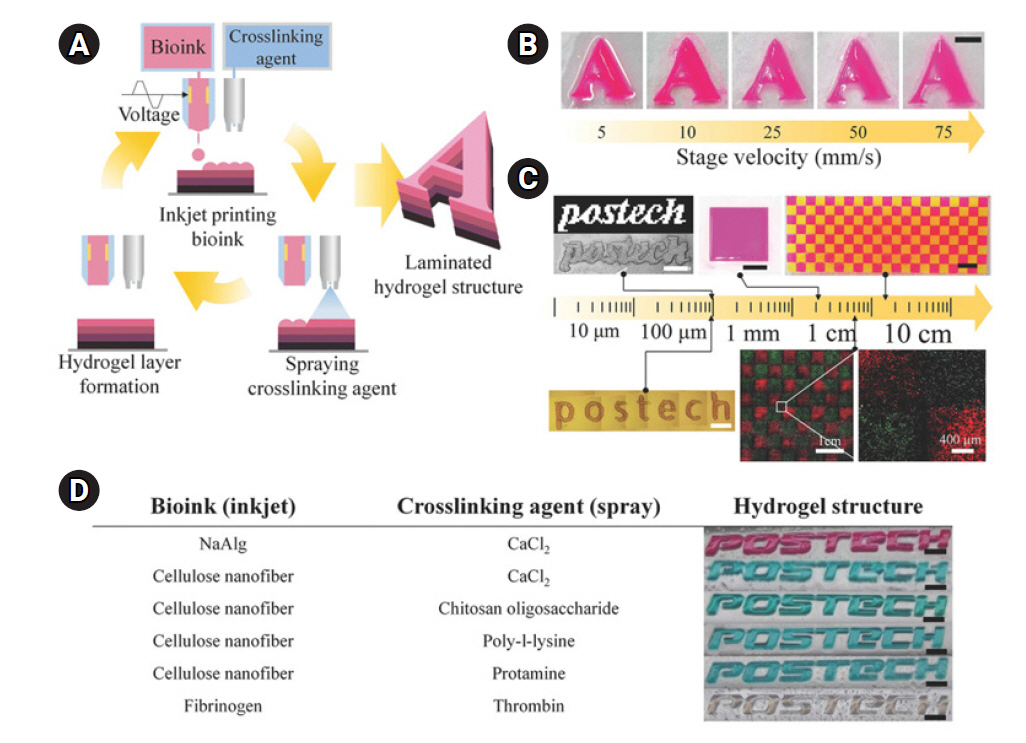
57. Jung S, Hutchings IM. The impact and spreading of a small liquid drop on a non-porous substrate over an extended time scale. Soft Matter 2012;8:2686-96.

58. Yoon S, Sohn S, Kwon J, Park JA, Jung S. Double-shot inkjet printing for high-conductivity polymer electrode. Thin Solid Films 2016;607:55-8.

60. Lee Y, Kwon J, Jung S, Kim W, Baek S, Jung S. Reliable inkjet contact metallization on printed polymer semiconductors for fabricating staggered TFTs. Appl Phys Lett 2020;116:153301.


61. Kwon J, Baek S, Lee Y, Tokito S, Jung S. Layout-to-bitmap conversion and design rules for inkjet-printed large-scale integrated circuits. Langmuir 2021;37:10692-701.


62. Singh M, Haverinen HM, Dhagat P, Jabbour GE. Inkjet printing-process and its applications. Adv Mater 2010;22:673-85.


63. Sumerel J, Lewis J, Doraiswamy A, Deravi LF, Sewell SL, Gerdon AE, et al. Piezoelectric ink jet processing of materials for medical and biological applications. Biotechnol J 2006;1:976-87.


64. Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res 2010;40:395-414.

65. Wilson WC Jr, Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec A Discov Mol Cell Evol Biol 2003;272:491-6.


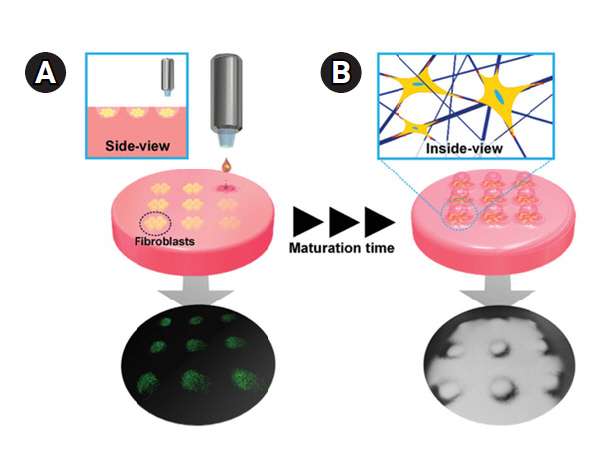
66. Nakamura M, Kobayashi A, Takagi F, Watanabe A, Hiruma Y, Ohuchi K, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng 2005;11:1658-66.


67. Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008;29:193-203.


68. Yusof A, Keegan H, Spillane CD, Sheils OM, Martin CM, O’Leary JJ, et al. Inkjet-like printing of single-cells. Lab Chip 2011;11:2447-54.


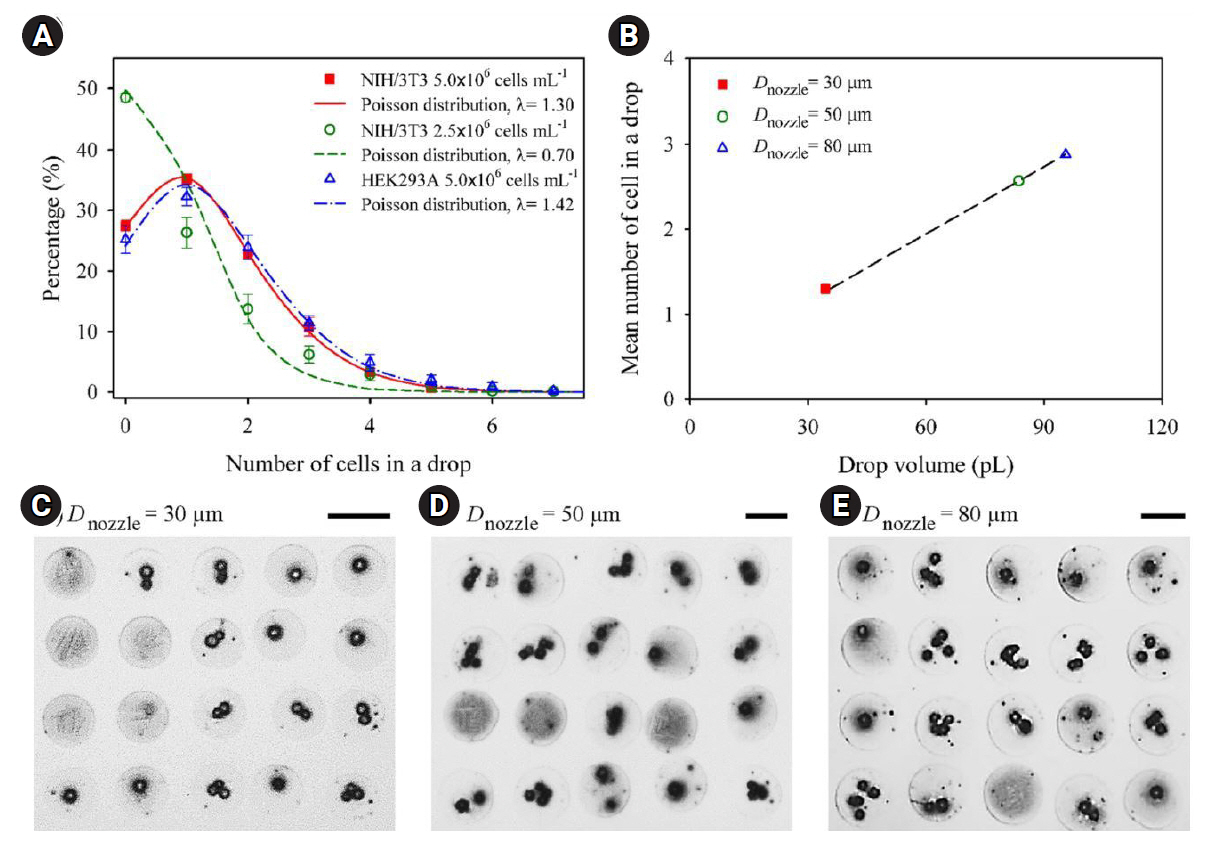
69. Yamaguchi S, Ueno A, Akiyama Y, Morishima K. Cell patterning through inkjet printing of one cell per droplet. Biofabrication 2012;4:045005.


70. Liberski AR, Delaney JT Jr, Schubert US. “One cell-one well”: a new approach to inkjet printing single cell microarrays. ACS Comb Sci 2011;13:190-5.


71. Chen F, Lin L, Zhang J, He Z, Uchiyama K, Lin JM. Single-cell analysis using drop-on-demand inkjet printing and probe electrospray ionization mass spectrometry. Anal Chem 2016;88:4354-60.


74. Baabdulla AA, Now H, Park JA, Kim WJ, Jung S, Yoo JY, et al. Homogenization of a reaction diffusion equation can explain influenza A virus load data. J Theor Biol 2021;527:110816.


76. Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng 2012;109:3152-60.


80. Lee HR, Park JA, Kim S, Jo Y, Kang D, Jung S. 3D microextrusion-inkjet hybrid printing of structured human skin equivalents. Bioprinting 2021;22:e00143.

83. Kang D, Lee Y, Kim W, Lee HR, Jung S. 3D pulmonary fibrosis model for anti-fibrotic drug discovery by inkjet-bioprinting. Biomed Mater 2022;18:015024.


88. Sellgren KL, Butala EJ, Gilmour BP, Randell SH, Grego S. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 2014;14:3349-58.


89. Humayun M, Chow CW, Young EW. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 2018;18:1298-309.


96. Doryab A, Taskin MB, Stahlhut P, Schröppel A, Wagner DE, Groll J, et al. A biomimetic, copolymeric membrane for cell‐stretch experiments with pulmonary epithelial cells at the air‐liquid interface. Adv Funct Mater 2021;31:2004707.














 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print