|
 |
| Organoid > Volume 3; 2023 > Article |
|
Abstract
Background
Research on salivary gland tissue is important, both for maintaining good oral health and for understanding the properties and functions of secretory cells with similar developmental mechanisms and functions. However, research on the pathogenesis of salivary gland disease and the development of therapeutic agents is very insufficient, and no studies have yet reported effective approaches to changes that occur according to the concentration of Matrigel, which is used to mimic the interactions between cells and the extracellular matrix, in three-dimensional organoid culture systems.
Methods
We aimed to identify the most suitable concentration of Matrigel for analyzing the morphology and differentiation patterns of organoids prepared using salivary submandibular gland-derived cells after inducing differentiation with or without a specific chemical, Y-27632.
Results
In both low and high Matrigel concentration conditions, morphological differences were found between the control group and the Y-27632 treatment group, and the budding structure was also significantly higher in the Y-27632 treatment group. However, more distinct patterns of differentiation appeared in the high-concentration condition.
To maintain good oral health, saliva is essential for a variety of reasons, including the protection of oral mucous membranes, the maintenance of teeth, and the digestion of starch. Sjögren's syndrome, and radiation treatment interfere with the function and production of the salivary glands, resulting in decreased quality of life due to the reduced secretion of salvia [1]. Poor oral health can lead to systemic disease and lower people's quality of life; thus, there is a need for governmental funding and attention in order to develop a mechanism for preventing and treating xerostomia.
The ROCK signaling pathway is regulated by Rho family GTPases and their downstream effector, ROCK, and is essential for cellular functions, including polarity, contractility, motility, proliferation, and apoptosis [2,3]. It has been reported that ROCK signaling directly affects apoptosis in in vitro cultured cells. ROCK-dependent hyperactivation of actin-myosin contractions in human embryonic stem cells induced apoptosis, and this was reduced by knockdown of ROCK1/2 and by the treatment of human embryonic stem cells with Y-27632, a ROCK inhibitor [4,5]. The viability of salivary gland stem cells (SGSCs) was enhanced and dissociation-induced cell death in SGSCs was inhibited when Y-27632 was administered through passage in spheroid culture. Survival and differentiation were also promoted in 3-dimensional (3D) culture of salivary gland-derived cells [6].
The extracellular matrix (ECM) provides structural support to cells, as well as establishing a microenvironment for them [7]. According to previous studies, the ECM plays a role in regulating cell survival and apoptosis [8-11]. When the anchoring ability of cells is damaged, an imbalance between the actin-myosin contractile force and the opposing force is induced, resulting in abnormal cell morphology and function [4,12-14]. An unbalanced state can cause cell death if it is maintained for a long period of time [15]. Accordingly, 3D organoids made from salivary gland submandibular gland cells (SGCs) were embedded in a low or high concentration of Matrigel to investigate how Matrigel affects the cell-ECM interaction in the 3D organoid environment. The expression levels of relevant genes were then measured after inducing differentiation with or without Y-27632.
Ethics statement: This study did not include human participants; therefore, it was exempt from institutional review board approval.
Five-week-old female mice (C57BL/6) were used. All animal experiments were carried out following the guidelines of the Institutional Animal Care and Use Committee of Seoul National University (approval number: SNU-210105-6-4).
Isolated submandibular glands were minced with a razor blade, and the homogenate was incubated in Hanks’ Balanced Salt Solution with CaCl2, MgSO4 (Welgene, Gyeongsan, Korea), together with 1 mg/mL hyaluronidase (Sigma, St. Louis, MO, USA) and 0.2% collagenase II (Thermo Fisher, Waltham, MA, USA). Mechanically and enzymatically dissociated cells were incubated in 60 mm dishes at 37°C for 1 hour. Cells were neutralized with Dulbecco’s modified Eagle’s medium (DMEM; Welgene) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), and centrifuged at 500×g for 3 minutes. After incubation, debris was filtered through 100 and 40 µm strainers (SPL Life Sciences Co., Ltd., Seoul, Korea) for single-cell isolation. The isolated cells were suspended in a basal medium composed of DMEM/F12 1:1 (v/v; Thermo Fisher), 20 ng/mL fibroblast growth factor-2 (Peprotech, Rocky Hill, NJ, USA), 20 ng/mL epithelial growth factor (Peprotech), 1% N-2 supplement (Thermo Fisher), 1% insulin (Cell Application, San Diego, CA, USA), 1 µM dexamethasone (Sigma), and 1% penicillin-streptomycin (Sigma). Cells were then cultured onto poly-HEMA-coated 60 mm culture dishes in a CO2 incubator (5% CO2 in humidified air) for 5 days.
The cells were harvested and prepared in a single-cell suspension. The total suspension (40 μL) was then mixed with 80 μL of growth factor reduced (GFR) Matrigel (Corning, NY, USA) to form the high concentration of Matrigel group, or with 40 μL of DMEM/F12 1:1 and 40 μL of GFR Matrigel to form low concentration of Matrigel group. The cells were seeded in Matrigel-pre-coated 48-well plates (SPL Life Sciences) to prepare SGC organoids. After solidifying the gels at 37°C for 20 minutes, basal medium was added, and SGC organoids were maintained for 4 days in a CO2 incubator (5% CO2 in humidified air). The basal medium was changed into differentiation medium composed of DMEM/F12 1:1 (v/v; Thermo Fisher), 1 mg/mL bovine serum albumin (Bovogen Biologicals Pty Ltd., Keilor East, Australia), 1% Insulin-Transferrin-Selenium (Thermo Fisher), 1% N-2 supplement (Thermo Fisher), 200 ng/mL fibroblast growth factor-1 (Peprotech), 20 ng/mL fibroblast growth factor-7 (Peprotech), 1 μM Y-27632 (Cayman Chemical, Ann Arbor, MI, USA), 1% penicillin-streptomycin (Sigma) for 8 days. The medium (200 µL/well) was added and changed every 2-3 days and maintained in a CO2 incubator (5% CO2 in humidified air). A total of 1 mg/mL of dispase (Life Technologies, Camarillo, CA, USA) was added and incubated at 37°C for 30 minutes to harvest the organoids. The percentage of spheroids with budding structures was measured by counting the number of spheroids with budding structures among the total number of spheroids.
Total RNA was extracted using a PureLinkTM RNA Mini Kit (Invitrogen, Camarillo, CA, USA). cDNA synthesis was performed M-MLV reverse transcriptase following the manufacturer’s protocol (M.biotech, Hanam, Korea). Real-time polymerase chain reaction (PCR) was performed using SYBR Pre-mix Ex Taq II (Takara, Tokyo, Japan) and a StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Cycling was performed for 30 seconds at 95°C, followed by 40 amplification cycles of 5 seconds at 95°C and 30 seconds at 60°C. Gapdh was used for normalization. The primer sequences for murine genes are listed in Table 1.
All data were presented as the mean±standard deviation, and the Student t-test, as well as one-way analysis of variance followed by Tukey’s post-hoc test, was performed to determine the significance of experimental differences. Statistical analysis were performed using Prism 7.0 (GraphPad, Boston, USA). All experiments were independently repeated at least 3 times. A p-value <0.05 was considered statistically significant.
Salivary gland organoids derived from submandibular glands were generated using the paradigm illustrated in Fig. 1. Suspensions of cells were induced to form compact aggregates and then embedded in Matrigel to mimic the in vivo environment of the salivary gland. The organoids were maintained for 12 days under specific conditions, using basal medium for 4 days and differentiation medium for 8 days. The organoids were harvested on day 12 after embedding, and a series of analyses was performed.
First, we observed SGC organoids embedded in low-concentration Matrigel to assess morphological and molecular changes associated with the differentiation of embedded structures. Budding structures appeared from day 5 after organoid culture (data not shown), and interestingly, Y-27632 induced significant cell growth and reduced apoptosis compared to the control group on day 10 and day 12 (i.e., after 6 and 8 days of differentiation medium treatment) (Fig. 2A). Moreover, Y-27632 significantly increased the number of spheroids with budding structures compared to the control group (13.43±1.683 and 73.37±6.736, respectively) (Fig. 2B). The mRNA expression level of the functional marker, Amy1, was significantly upregulated in spheroids of the treated cells. However, the expression levels of the acinar marker, Mist 1, and ductal marker, Krt 8 showed a small but significant decrease. Another acinar marker, Aqp 5, and ductal progenitor marker, Ascl3, showed no significant difference between the 2 groups of cells (Fig. 2C). These results suggest that Y-27632 induces differentiation into SGC organoids in a low Matrigel concentration-based 3D culture system in terms of morphology, but whether this leads to clear differentiation into the acinar or ductal lineage is not known exactly.
Next, we observed SGC organoids embedded in high-concentration Matrigel to assess morphological and molecular changes associated with the differentiation of embedded structures compared to the low Matrigel concentration condition. As in the low-concentration Matrigel, budding structures appeared from day 5 after organoid culture (data not shown). Also, Y-27632 induced significant cell growth compared to the control group on day 10 and day 12 (i.e., after 6 and 8 days of differentiation medium treatment). Interestingly, the size of organoids in the Y-27632 treatment group was significantly smaller compared to those embedded in low-concentration Matrigel, and the budding structure was better displayed in the bright field (Fig. 3A). As noted above, Y-27632 significantly increased the number of spheroids with budding structures compared to the control group (21.5±2.784 and 75.57±2.385, respectively) (Fig. 3B). Similar to the morphology, mRNA expression levels showed a difference from the low Matrigel concentration condition. The mRNA expression of Amy1, Mist 1 and Ascl3 showed significant increases compared to the control group. However, as in the low Matrigel concentration condition, Aqp5 did not show a significant difference between the 2 groups of cells, and the expression level of Krt8 decreased slightly (Fig. 3C). These results suggest that Y-27632 clearly induces differentiation of SGC organoids in a high-concentration Matrigel compared to a low-concentration condition.
In recent years, advances in 3D organoid culture have allowed stem cell-based organoid culture to be established over an extended period of time [16,17]. Researchers have demonstrated that organoids can simulate in vivo tissue morphogenesis, making them useful for studying the development and regeneration of organs. However, salivary gland organoids have not been extensively studied in relation to adult tissue morphogenesis. Therefore, in this study, we investigated a salivary gland organoid culture system that could recapitulate the morphogenesis of salivary gland organoids and analyzed the changes in the 3D organoid culture system according to the concentration of Matrigel used to mimic the interaction between cells and ECM.
It has been reported that Y-27632 increased the population of proacinar cells in salivary gland organoids [18]. Accordingly, more budding structures appeared in the Y-27632 treated group than in the control group when maintaining the organoid in the differentiation medium after embedding the cells in Matrigel, which is similar to the results of previous studies [6,18]. Y-27632 has been shown to block the effect of YAP/TAZ through the Hippo pathway, which is modulated by RHO-ROCK signaling [19,20], which means that sustained activation of the pathway induced by YAP degradation promotes the terminal differentiation of mature cell types [21]. In contrast, various studies have shown that a stiff ECM and high degree of cell spreading induce higher cellular tension and subsequently upregulate mechanotransducers such as RhoA and YAP/TAZ [22]. This seems to be the reason why the higher concentration of Matrigel with the treatment of Y-27632 was more effective than the lower concentration of Matrigel.
We performed various analyses to determine whether the cell-ECM interactions mimicked through Matrigel had an effect on budding structure and differentiation into acinar lineages. For organoids embedded in high-concentration Matrigel, the number of spheroids showing budding structures was similar, but the size of the spheroids made was smaller than those of other organoids (data not shown). Furthermore, differentiation into the acinar lineage was confirmed at the mRNA expression level. This is considered to be because ECM properties, including matrix stiffness, regulate cell responses to the microenvironment, and this response is mimicked to a lesser extent when the concentration of Matrigel is low because organoids are maintained in a floating form rather than being embedded. However, the expression of Aqp5, an acinar marker, was not confirmed in both concentration conditions, so further research is needed, and additional research for analyzing whether differences in mRNA expression lead to changes in protein levels is also required.
In conclusion, we demonstrate that the concentration of Matrigel in the 3D organoid culture system has a significant effect on mimicking the actual in vivo environment. This study will help make it possible to develop original technology that can overcome the current situation, in which research on the pathogenesis of salivary gland diseases and the development of therapeutic agents is very insufficient.
NOTES
Fig. 1.
Schematic diagram of the methods used for organoid formation. SGC, submandibular gland cell; 3D, 3-dimensional.
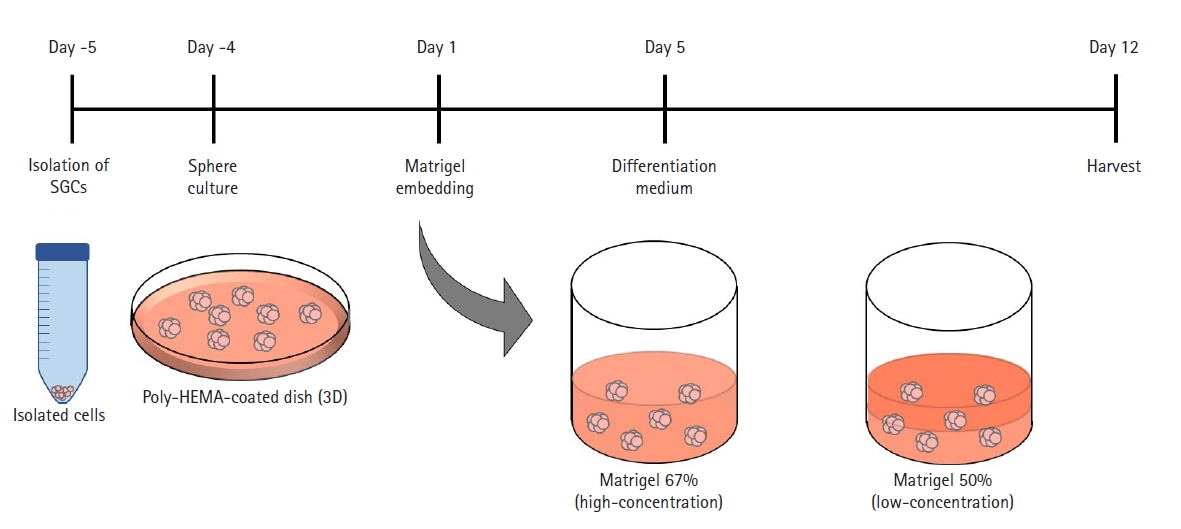
Fig. 2.
Effects of low-concentration Matrigel on the generation of submandibular gland cell (SGC) organoid structures. (A) The morphology of SGC-derived spheroids generated after embedding in a low concentration of Matrigel in the presence and absence of Y-27632. Images were obtained on days 10 and 12 after embedding. Scale bar=100 μm. (B) Percentage of spheroids with budding structures. (C) Expression of acinar- and duct-specific markers. Their mRNA levels were measured by extraction of RNA from organoids grown in the presence of Matrigel (n=8) in technical triplicates with real-time quantitative polymerase chain reaction.
*p<0.05, **p<0.01, ***p<0.001.
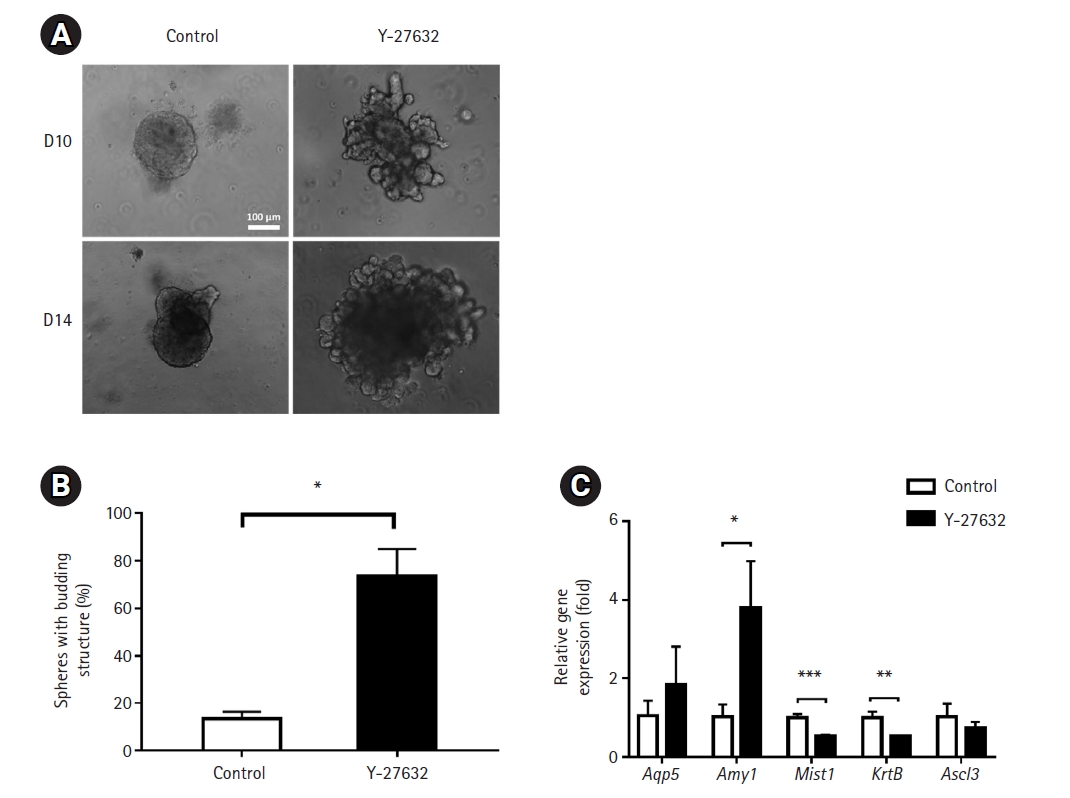
Fig. 3.
Effects of high-concentration Matrigel on the generation of submandibular gland cell (SGC) organoid structures. (A) The morphology of SGC-derived spheroids generated after embedding in a high concentration of Matrigel in the presence and absence of Y-27632. Images were obtained on days 10 and 12 after embedding. Scale bar=100 μm. (B) Percentage of spheroids with budding structures. (C) Expression of acinar- and duct-specific markers. Their mRNA levels were measured by extraction of RNA from organoids grown in the presence of Matrigel (n=8) in technical triplicates with real-time quantitative polymerase chain reaction.
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
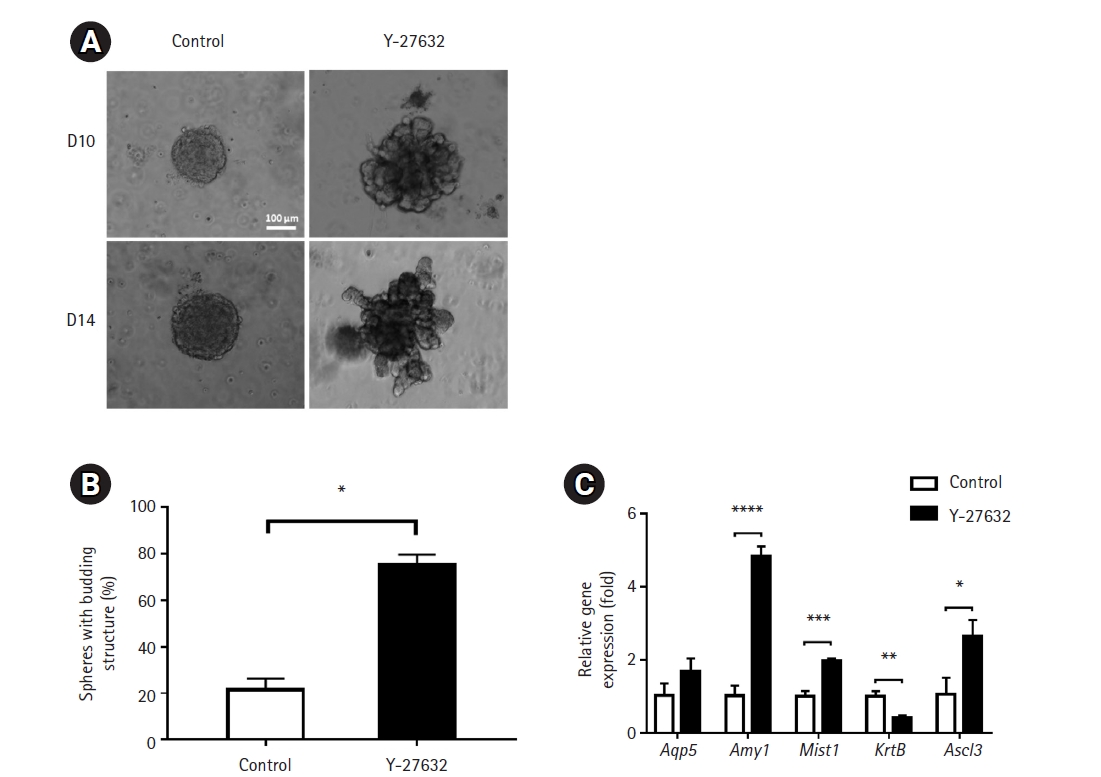
Table 1.
Murine primer sequences used for quantitative polymerase chain reaction (5’→3’)
References
1. Ikeura K, Kawakita T, Tsunoda K, Nakagawa T, Tsubota K. Characterization of long-term cultured murine submandibular gland epithelial cells. PLoS One 2016;11:e0147407.



3. Pertz O. Spatio-temporal Rho GTPase signaling: where are we now? J Cell Sci 2010;123(Pt 11):1841-50.



4. Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 2010;7:240-8.



5. Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 2010;7:225-39.


6. Kim K, Min S, Kim D, Kim H, Roh S. A Rho Kinase (ROCK) inhibitor, Y-27632, inhibits the dissociation-induced cell death of salivary gland stem cells. Molecules 2021;26:2658.



7. Sapudom J, Pompe T. Biomimetic tumor microenvironments based on collagen matrices. Biomater Sci 2018;6:2009-24.


8. Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995;267:891-3.



9. Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci U S A 1996;93:3509-13.



10. Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 1997;276:1425-8.


11. Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, et al. Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int 1998;54:1188-96.


12. Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 2007;8:633-44.



13. Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science 2008;322:1502-5.


14. Okeyo KO, Adachi T, Sunaga J, Hojo M. Actomyosin contractility spatiotemporally regulates actin network dynamics in migrating cells. J Biomech 2009;42:2540-8.


15. Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005;435:365-9.




17. Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell 2016;38:590-600.


18. Koslow M, O’Keefe KJ, Hosseini ZF, Nelson DA, Larsen M. ROCK inhibitor increases proacinar cells in adult salivary gland organoids. Stem Cell Res 2019;41:101608.



19. Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol 2012;19:955-62.


20. Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev Biol 2014;394:142-55.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 945 View
- 21 Download
- ORCID iDs
-
Sangho Roh

https://orcid.org/0000-0001-8082-6459 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print