|
 |
| Organoid > Volume 4; 2024 > Article |
|
Abstract
NOTES
Funding
We thank Hyunjin Lee for the illustration. In-Hyun Park was partly supported by NIH (R01MH118344-01A1). W.Y was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education(2021R1A6A3A14043824).
Fig. 1.

Fig. 2.
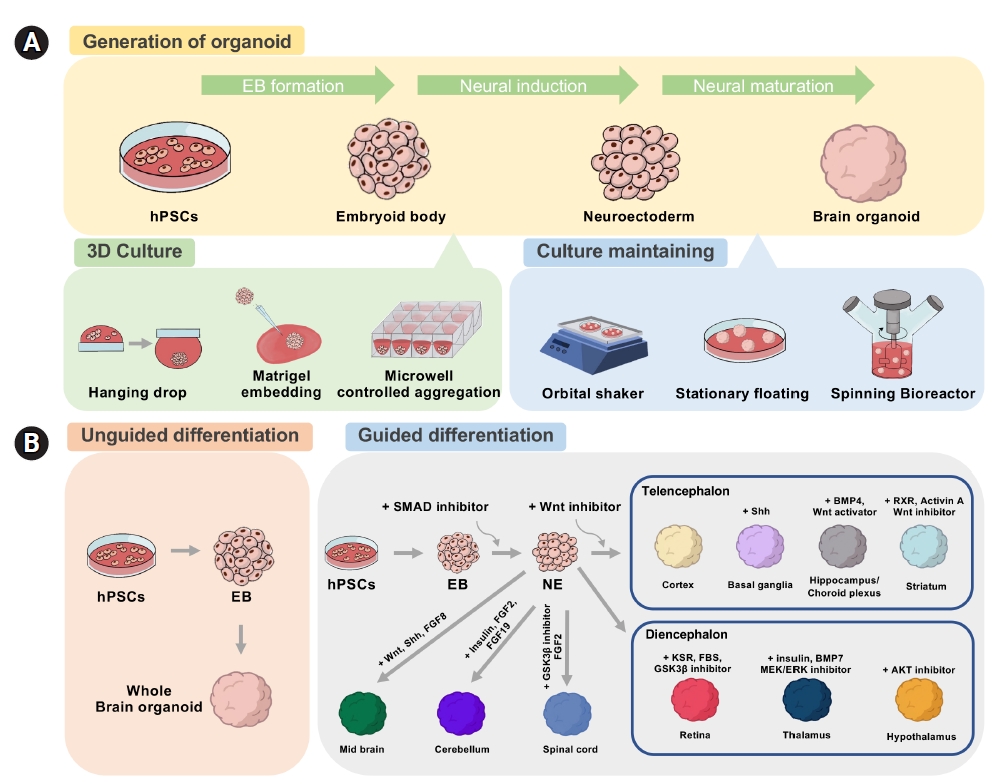
Fig. 3.
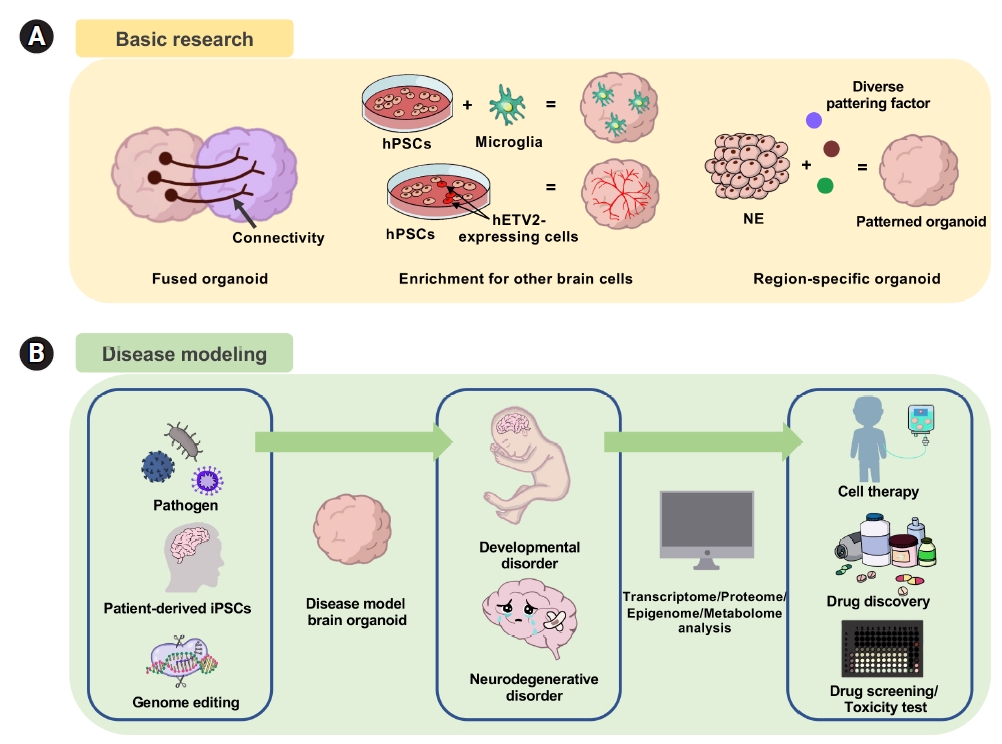
Table 1.
| Methodology | Type of brain region | Type of organoid | Extrinsic factors | Culture method | Extracellular scaffolding | Reference |
|---|---|---|---|---|---|---|
| Unguided method | Whole brain | Cerebral organoid | Low bFGF | Spinning bioreactor | Matrigel | [51] |
| Cerebral organoid | Low bFGF | Spinning bioreactor | Matrigel | [52] | ||
| Forebrain | Cerebral organoid | Low bFGF | Spinning bioreactor | Matrigel | [53] | |
| Cerebral organoid | Low bFGF | Spinning bioreactor | Matrigel | [54] | ||
| Cerebral organoids | Low bFGF | Spinning bioreactor | Matrigel | [55] | ||
| Guided method | Cerebral cortex | Cortical spheroid | Dorsomorphin, SB-431542, FGF2, EGF | Stationary floating | - | [56,57] |
| Cortical neuroepithelium | IWR1e, SB431542 | Stationary floating | Matrigel | [58] | ||
| Cortical organoid | Noggin, hDkk1, FGF2 | Stationary floating | Matrigel on dish | [59] | ||
| Cortical organoid | SB-431542, LDN-193189, XAV939 | Orbital shaker | - | [60] | ||
| Forebrain organoid | IWR1e, SB431542 | Spinning bioreactor | Matrigel | [61] | ||
| Forebrain organoid | Wnt3a, Dorsomorphin, A83-01, CHIR99021, SB431542 | Miniaturized Spinning Bioreactor | Matrigel | [62] | ||
| Ventral forebrain | Ventral organoid | IWP-2, SAG | Orbital shaker | Matrigel | [63] | |
| Subpallium spheroids | Dorsomorphin, SB431542, IWP-2, SAG | Stationary floating | - | [64] | ||
| Medial ganglionic eminence | MGE organoid | SB431542, LDN193189, XAV939, Shh, Purmorphamine | Orbital shaker | - | [60] | |
| Lateral ganglionic eminence | Striatal organoid | Dorsomorphin, SB431542, Activin A, IWP-2, SR11237, DAPT | Stationary floating | - | [65] | |
| Hippocampus | Hippocampal primordium-like tissue | SB431542, IWR1, CHIR99021, and BMP4 | Stationary floating | - | [66] | |
| Hippocampal Spheroids | SB-431542, LDN-193189, XAV939, Cyclopamine, CHIR99021 | Stationary floating | - | [67] | ||
| Hippocampal organoid | Dorsomorphin, A83-01, SB-431542, CHIR-99021, BMP7, | Orbital shaker | Matrigel | [68] | ||
| Choroid plexus | Choroid plexus-like tissue | SB431542, IWR1, CHIR99021, and BMP4 | Stationary floating | - | [66] | |
| Choroid plexus organoid | LDN-193189, SB-431542, IWP-2, CHIR-99021, BMP7 | Orbital shaker | Matrigel | [68] | ||
| Choroid plexus organoid | BMP4, CHIR-99021 | Spinning bioreactor | Matrigel | [69] |
Table 2.
|
Disease modeling |
Type of organoid | Disease phenotype of organoid | Reference | |
|---|---|---|---|---|
| Disease type | Substance/gene | |||
| Microcephaly | Zika virus | Forebrain organoid | Decrease of neuronal cell-layer volume, resembling microcephaly | [62] |
| Forebrain organoid | Disruption of cortical neurogenesis | [83] | ||
| Cerebral organoid | Perturbed cell fate, a reduction in organoid volume | [84] | ||
| Cerebral organoid | Reduction of proliferative zones, disrupted cortical layers | [85] | ||
| Cerebral organoid | Neural progenitor apoptosis, growth restriction | [86] | ||
| Cerebral organoid | Reduced size and viability, programmed cell death responses. | [87] | ||
| SARS-CoV-2 virus | Cortical organoid | - | [88] | |
| Cerebral organoid | Neuronal cell death, aberrant Tau localization, | [89] | ||
| Choroid plexus organoid | disruption of blood-CSF barrier | [90] | ||
| HCMV virus | Cerebral Organoid | Reduction in organoid volume, degeneration of β-tubulin III integrity | [91] | |
| Alzheimer's disease | APP/PSN1 | Hippocampal spheroid | Loss of synaptic proteins, increased ratio of intracellular and extracellular Aβ42/Aβ40 peptides | [67] |
| APP | Neocortex | Aβ aggregation, hyperphosphorylated tau protein, endosome abnormalities | [92] | |
| PSN1 | Cerebral organoid | Higher production of the Aβ protein, increased tau phosphorylation | [93] | |
| APOE4 | Cerebral organoid | Increased levels of Aβ and phosphorylated tau | [94] | |
| Rett syndrome | MeCP2 | Forebrain Organoid | Lower expression of neural progenitor, defect of electrophysiological activity | [95] |
| Cortical organoid, MGE organoid | Dysregulated gene in neurons and glial cells, abnormal transcription related to synaptic transmission | [96] | ||
| Autism Spectrum Disorders, | FOXG1 | Telencephalic organoid | Accelerated cell cycle, overproduction of GABAergic inhibitory neurons | [59] |
| CDK5RAP2 | Cerebral organoid | Premature neuronal differentiation | [45] | |
| PTEN | Cerebral organoid | Delayed neuronal differentiation, expanded VZ and oSVZ, surface expansion and folding | [97] | |
| CHD8 | Cerebral organoid | Dysregulated Wnt/β-catenin signaling, GABAergic interneuron related gene | [98] | |
| Miller-Dieker syndrome | PAFAH1B1 | Cerebral organoid | Increase apoptosis/vertical spindle orientation, prolonged mitosis | [99] |
| Timothy syndrome | CACNA1C | Cortical and subpallium spheroid | Abnormal migratory saltation | [64] |
| Fragile X syndrome | FMR1 | Forebrain organoid | Dysregulated neurogenesis, neuronal maturation and neuronal excitability. | [100] |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HCMV, human cytomegalovirus; APP, amyloid precursor protein; PSN1, presenilin 1; APOE4, apolipoprotein E4; MeCP2, methyl-CpG binding protein 2; FOXG1, forkhead box G1; PTEN, phosphatase and tensin homolog; CDK5RAP2, CDK5 regulatory subunit associated protein 2; CHD8, chromodomain-helicase-DNA-binding protein 8; PAFAH1B1, platelet activating factor acetylhydrolase 1b regulatory subunit 1; CACNA1C, L-type calcium channel Cav1. 2; FMR1, fragile X messenger ribonucleoprotein 1.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,485 View
- 25 Download
- ORCID iDs
-
Woo Sub Yang

https://orcid.org/0000-0002-2358-8287Ferdi Ridvan Kiral

https://orcid.org/0000-0002-7006-9332In-Hyun Park

https://orcid.org/0000-0001-7748-1293 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print